Chelating Agents
In coordination compounds, the ligand attaches to the central metal ion through the donor atom. A bidentate ligand attaches to the metal ion in two places (two donor atoms), and a polydentate ligand attaches to the central atom in three or more locations. A ligand that has two or more donor atoms is called a chelating agent.
Examples:
1. Ethylenediamine
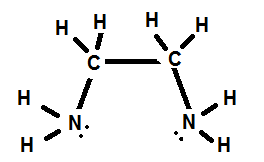
In this example the ligand can attach to the metal ion at the site of both N atoms.
2. Terpyridyl
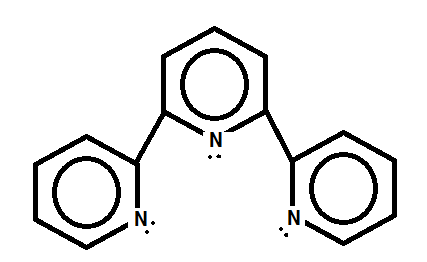
In this polydentate ligand, the molecule can have three donor atoms.
Examples:
1. Ethylenediamine

In this example the ligand can attach to the metal ion at the site of both N atoms.
2. Terpyridyl

In this polydentate ligand, the molecule can have three donor atoms.
|
Related Links: Chemistry Transition Metals Coordination Compounds Naming Coordination Compounds |
To link to this Chelating Agents page, copy the following code to your site:
