Coordination Compounds
Coordination compounds refer to a group of compounds in which a central metal ion is bonded to electron rich ligands. The central metal ion is typically a transition metal which will have available d-orbitals that can accept an electron, or pair of electrons from the ligand. The electron pair being donated and received constitutes the reaction as a Lewis acid-base reaction.
When the ligand bonds to the central metal ion, the atom that attaches to the metal is known as the donor atom. The number of ligands that attaches is known as the coordination number which is larger than the oxidation number of the central metal ion. For example, the [Ag(NH3)2]+ complex ion has the silver metal ion with a +1 oxidation number and two NH3 ligands.
Examples:
1. The [Ag(NH3)2]+ complex ion. Notice how the name changes from coordination compound to coordination complex.
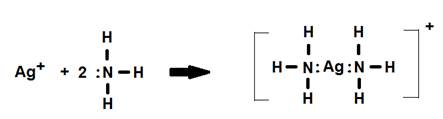
In this example the coordination number (number of ligands) is two and N is the donor atom.
2. The [Zn(NH3)4]2+ complex ion.
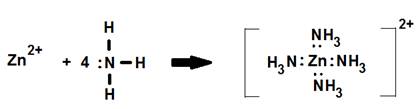
In this example the coordination number is four while the surrounding NH3 ligands form a tetrahedral around the central Zn.
When the ligand bonds to the central metal ion, the atom that attaches to the metal is known as the donor atom. The number of ligands that attaches is known as the coordination number which is larger than the oxidation number of the central metal ion. For example, the [Ag(NH3)2]+ complex ion has the silver metal ion with a +1 oxidation number and two NH3 ligands.
Examples:
1. The [Ag(NH3)2]+ complex ion. Notice how the name changes from coordination compound to coordination complex.

In this example the coordination number (number of ligands) is two and N is the donor atom.
2. The [Zn(NH3)4]2+ complex ion.

In this example the coordination number is four while the surrounding NH3 ligands form a tetrahedral around the central Zn.
|
Related Links: Chemistry Transition Metals Chelating Agents Naming Coordination Compounds |
To link to this Coordination Compounds page, copy the following code to your site:
