Alkane Structure and Bonding
Alkane is a term used to describe a hydrocarbon that contains only single carbon to carbon bonds. The single bonds allow the carbon atoms to bond with as many hydrogen atoms as allowed by carbon's bonding principles. Because of this, alkanes are referred to as "saturated" hydrocarbons. They have the general formula CnH2n+2, where n is any integer. For example, if an alkane contained 5 carbon atoms the formula for the alkane would be C5H12.
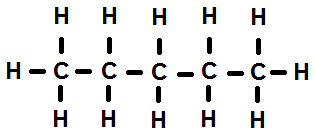
Alkanes can form where the carbon atoms are connected in a row, like the example of pentane (C5H12) above. This is referred to as a normal alkane. They can also form a branched chain, and even a cycloalkane in which the atoms form a ring. In a cycloalkane the general formula is CnH2n.
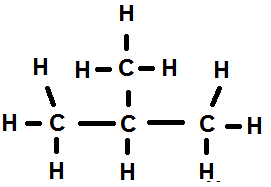
1. Branched chain alkane: Isobutane
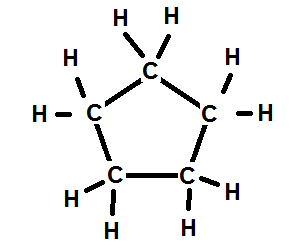
2. Cycloalkane: Cyclopentane
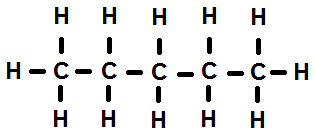
Alkanes can form where the carbon atoms are connected in a row, like the example of pentane (C5H12) above. This is referred to as a normal alkane. They can also form a branched chain, and even a cycloalkane in which the atoms form a ring. In a cycloalkane the general formula is CnH2n.
1. Branched chain alkane: Isobutane

2. Cycloalkane: Cyclopentane

|
Related Links: Chemistry Organic Chemistry Dipole-Dipole Interactions Free Radicals |
To link to this Alkane Structure and Bonding page, copy the following code to your site:
