Dipole-Dipole Interactions
Forces of attraction that draw molecules together are called intermolecular forces. The three main intermolecular forces are London dispersion forces, dipole-dipole interactions and hydrogen bonding. Dipole-dipole interactions are caused by the attraction of two polar molecules.
If a molecule has an area of positive charge and an area of negative charge it is said to be polar or have a dipole moment. This is typically caused by the atoms in the molecule having stronger (higher electronegativity) or weaker (lower electronegativity) attractions for the electrons in the bond(s). The atom that has the stronger attraction will pull the electrons in the bond away from the atom with the weaker attraction. This will cause one atom to have an area with more electrons and thus carry a partial negative charge. The atom with an area of electron deficiency will carry a partial positive charge. A neighboring molecule with a similar disposition can attract to this molecule creating a dipole-dipole interaction.
Examples:
1. The dipole-dipole interaction of carbon monoxide.
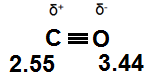
In this molecule the electronegativity value of the oxygen (3.44) is larger than the value for carbon (2.55). The electrons in the triple bond are pulled closer to the oxygen side of the molecule creating areas of positive and negative charge.
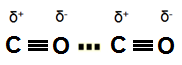
Two neighboring carbon monoxide molecules can have a dipole-dipole interaction.
2. The dipole-dipole interaction of ICl.
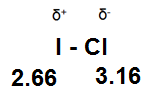
In this molecule the uneven sharing of electrons creates a dipole moment with the chlorine carrying a partial negative charge while the iodine carries a partial positive charge.

This represents the dipole-dipole interaction of two ICl molecules.
If a molecule has an area of positive charge and an area of negative charge it is said to be polar or have a dipole moment. This is typically caused by the atoms in the molecule having stronger (higher electronegativity) or weaker (lower electronegativity) attractions for the electrons in the bond(s). The atom that has the stronger attraction will pull the electrons in the bond away from the atom with the weaker attraction. This will cause one atom to have an area with more electrons and thus carry a partial negative charge. The atom with an area of electron deficiency will carry a partial positive charge. A neighboring molecule with a similar disposition can attract to this molecule creating a dipole-dipole interaction.
Examples:
1. The dipole-dipole interaction of carbon monoxide.
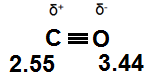
In this molecule the electronegativity value of the oxygen (3.44) is larger than the value for carbon (2.55). The electrons in the triple bond are pulled closer to the oxygen side of the molecule creating areas of positive and negative charge.

Two neighboring carbon monoxide molecules can have a dipole-dipole interaction.
2. The dipole-dipole interaction of ICl.

In this molecule the uneven sharing of electrons creates a dipole moment with the chlorine carrying a partial negative charge while the iodine carries a partial positive charge.

This represents the dipole-dipole interaction of two ICl molecules.
|
Related Links: Chemistry Organic Chemistry Free Radicals Hydrogen Bonding |
To link to this Dipole-Dipole Interactions page, copy the following code to your site:
