Free Radicals
A free radical is a term used in chemistry to define a particle that has unpaired valence electrons. This often leads to an odd number of electrons unless two unpaired electrons occur. Free radicals are highly reactive due to the availability of space in a single electron orbital. This will have varied affects depending on the situation.
One positive affect of a free radical is that it can be formed and consumed in a reaction mechanism. This will help facilitate a chemical reaction. On the other hand, free radicals can cause unwanted reactions in cells and tissues. Often times a reaction with a free radical will produce more free radicals and a chain reaction will start. This can prove to be very damaging because it will interrupt the behavior of a normal cell.
Examples of Free Radicals
Note the presence of a free radical by the placement of a dot on the right side of the formula.
1. The hydroxyl radical (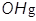 )
)
The hydroxyl radical can be described as a water molecule with only one hydrogen atom. It is particularly damaging to cells and tissue.
2. Nitric oxide (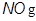 )
)
Nitric oxide (or nitrogen monoxide) is a free radical produced by combustion of air particles.
One positive affect of a free radical is that it can be formed and consumed in a reaction mechanism. This will help facilitate a chemical reaction. On the other hand, free radicals can cause unwanted reactions in cells and tissues. Often times a reaction with a free radical will produce more free radicals and a chain reaction will start. This can prove to be very damaging because it will interrupt the behavior of a normal cell.
Examples of Free Radicals
Note the presence of a free radical by the placement of a dot on the right side of the formula.
1. The hydroxyl radical (
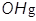 )
)The hydroxyl radical can be described as a water molecule with only one hydrogen atom. It is particularly damaging to cells and tissue.
2. Nitric oxide (
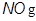 )
)Nitric oxide (or nitrogen monoxide) is a free radical produced by combustion of air particles.
|
Related Links: Chemistry Organic Chemistry Hydrogen Bonding Intermolecular Forces |
To link to this Free Radicals page, copy the following code to your site:
