Enduring Understanding 4.B.2: Boltzmann & Arrhenius
- To overcome the energy barrier of a reaction, a particle must have sufficient energy.
- Reaction mixtures contain a very large number of particles with differing amounts of energy.
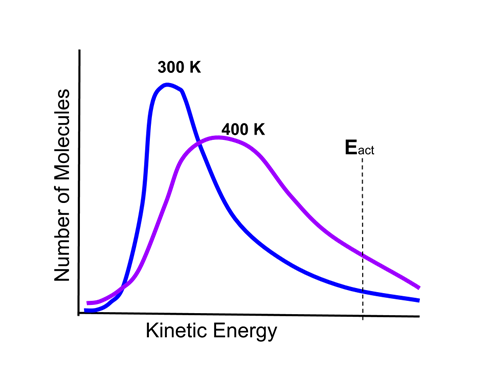
- The distribution of energies of particles can be described by the Maxwell-Boltzmann thermal distribution of particle energies. This is illustrated in the following graphic:
- There are two curves on the graph, one representing a sample of reactant gas molecules at a temperature of 300 K, and the other the same sample at a temperature of 400 K.
- There is also an activation energy threshold of a reaction, Eact, indicated.
- Molecules with energy above Eact will have sufficient energy to undergo a reaction. As the graph shows, there are more molecules above Eact at 400 K than at 300 K, therefore the reaction will occur more rapidly at the higher temperature
- The Arrhenius equation is a mathematical function that gives the rate of a reaction, as a function of the activation energy Ea and the temperature T.
- A and R are constants.
To link to this Boltzmann & Arrhenius page, copy the following code to your site:
