Enduring Understanding 4.B: Reaction Mechanism
- Reactions proceed at a molecular level, through elementary steps.
- These elementary steps can be unimolecular, or can involve collisions between two or more molecules.
- In a bimolecular (or higher order) elementary step, the collision must have both sufficient energy and be in a favorable orientation for the reaction to happen.
- The order of an elementary step depends on the number of molecules involved - a unimolecular step is first order, a bimolecular collision is second order, etc...
- Elementary steps involving collisions between three or more particles are rare.
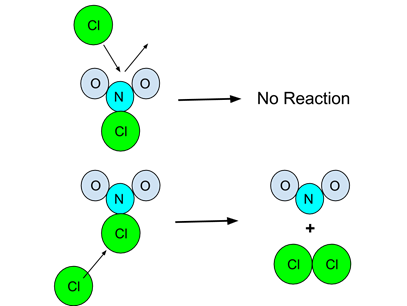
- Example: The following particulate diagram illustrates the elementary reaction
- The upper diagram shows a collision between a NO2Cl molecule and a Cl atom. However, the orientation of the collision is not correct and no reaction occurs.
- The lower diagram shows a collision, but in a more suitable orientation. The reaction then occurs.
- Because it is a bimolecular collision, the rate law of this elementary reaction would be k[Cl][NO2]
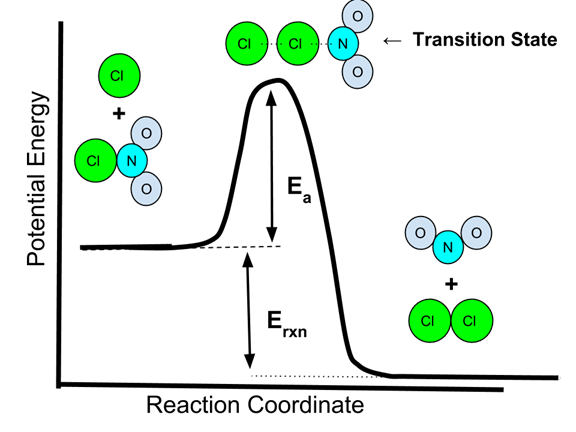
- Here is an energy diagram of the NO2Cl + Cl → NO2 + Cl2 reaction.
- The two colliding species must have sufficient energy to overcome the activation energy barrier, Ea.
- The high point in the energy curve represents the transition state, or activated complex, where bonds between the two Cl atoms and the NO2 have partially formed, partially broken bonds.
- The energy released by the reaction is Erxn. It does not depend on Ea.
|
Related Links: Chemistry Chemistry Quizzes AP Chemistry Notes Reaction Rates |
To link to this Reaction Mechanism page, copy the following code to your site:
