Mass Spectroscopy
One of the ways scientists can help identify an unknown sample is to use mass spectroscopy. As its name implies, the mass spectrometer is useful in identifying the mass and mass fragments of the sample. The masses can then be compared to a database of known compounds to try and identify the sample.
How it Works
When the sample is placed into a mass spectrometer it is bombarded by a high energy stream of electrons. The electrons break the sample into smaller fragments. Many of the fragments have a positive charge due to the loss of an electron during bombardment. The fragments are then passed through a magnetic field where they are separated (positive fragments from neutral fragments). The positive fragments are the only ones affected by the magnetic field and therefore are the only ones measured. The results are then graphed and used for comparison.
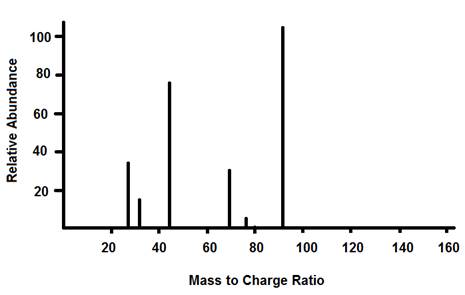
Example of Graphed Results
How it Works
When the sample is placed into a mass spectrometer it is bombarded by a high energy stream of electrons. The electrons break the sample into smaller fragments. Many of the fragments have a positive charge due to the loss of an electron during bombardment. The fragments are then passed through a magnetic field where they are separated (positive fragments from neutral fragments). The positive fragments are the only ones affected by the magnetic field and therefore are the only ones measured. The results are then graphed and used for comparison.
Example of Graphed Results

|
Related Links: Chemistry Organic Chemistry Physical Properties of Alkanes Physical Properties of Alkenes |
To link to this Mass Spectroscopy page, copy the following code to your site:
