Physical Properties of Alkanes
An alkane is a hydrocarbon that contains only carbon to carbon single bonds. They can be referred to as aliphatic compounds which stem from the Greek word aleiphas, meaning fat. This is due to the fact that animal fats are often composed of long chain alkanes.
Alkanes, in general, are not very reactive molecules. They show little reactivity to most organic reagents but do react with a few substances, most notably oxygen. Because of this, the term paraffin is used to refer to an alkane meaning "little affinity".
The reaction of alkanes with oxygen is one of the most important reactions in society. Alkanes will burn in the presence of oxygen to produce carbon dioxide and water. The reactions give off a tremendous amount of energy and alkanes (as well as many hydrocarbons) are used as fuel sources because of this. Natural gas (methane) for example is used to heat homes in the winter and in gas ovens.
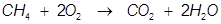
The reaction of natural gas (CH4) with oxygen.
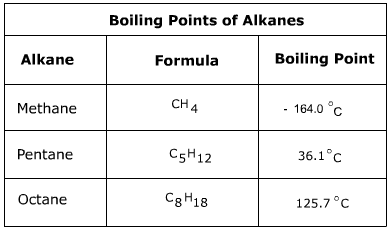
Alkanes are nonpolar molecules that have only London dispersion forces as a means to attract to one another. Because of this they have relatively low melting and boiling points. As the size of the alkane increases, however, so does the strength of the dispersion force and the melting and boiling points will increase.
Alkanes, in general, are not very reactive molecules. They show little reactivity to most organic reagents but do react with a few substances, most notably oxygen. Because of this, the term paraffin is used to refer to an alkane meaning "little affinity".
The reaction of alkanes with oxygen is one of the most important reactions in society. Alkanes will burn in the presence of oxygen to produce carbon dioxide and water. The reactions give off a tremendous amount of energy and alkanes (as well as many hydrocarbons) are used as fuel sources because of this. Natural gas (methane) for example is used to heat homes in the winter and in gas ovens.
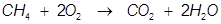
The reaction of natural gas (CH4) with oxygen.
Alkanes are nonpolar molecules that have only London dispersion forces as a means to attract to one another. Because of this they have relatively low melting and boiling points. As the size of the alkane increases, however, so does the strength of the dispersion force and the melting and boiling points will increase.

|
Related Links: Chemistry Organic Chemistry Physical Properties of Alkenes Spectroscopy |
To link to this Physical Properties of Alkanes page, copy the following code to your site:
