Enduring Understanding 6.A: Equilibrium Problems
- Sample Question 1: A chemical reaction will always be spontaneous (thermodynamically favored) under which conditions?
- Answer: (a), Exothermic with an increase in entropy
- Thermodynamic favorability of a chemical reaction is determined by the Gibbs free energy,
- If ΔG < 0, the reaction is spontaneous.
- Therefore, if ΔH is negative (reaction is exothermic) and ΔS is positive (increase in entropy) then ΔG must always be negative.
- Sample Question 2: Consider the reaction X(g) + Y(g) ⇆ Z(g), with K = 10 at 25 °C
- Which of the above samples are at equilibrium?
- Answer: (c), Samples 1 and 2
- The equilibrium constant is determined by K=[Z]/[X][Y]. If [Z]/[X][Y] = 10, the sample is at equilibrium. In both samples 1 and 2, [Z]/[X][Y] is 10, so they are both at equilibrium. In samples 3 and 4, [Z]/[X][Y] is 5, and the samples are not at equilibrium.
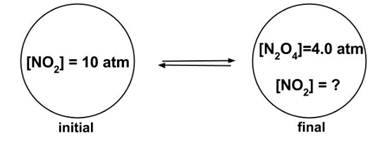
- Sample Question 3: Consider the reaction 2 NO2(g) ⇆ N2O4 (g). A rigid container is pressurized with 10 atm NO2, then allowed to come to equilibrium with a final concentration of 4.0 atm N2O4. What is Kp?
- Answer: (a), 1
- The problem can be solved as follows:
- Two equivalents NO2 are used to form 1 equivalent N2O4, so at 4.0 atm N2O4 the amount of NO2 remaining will be (10 - 2 x 4.0) or 2.0 atm.
- So the equation for K is
a) Exothermic, with an increase in entropy
b) Exothermic, with a decrease in entropy
c) Endothermic, with a decrease in entropy
d) Endothermic, with an increase in entropy
b) Exothermic, with a decrease in entropy
c) Endothermic, with a decrease in entropy
d) Endothermic, with an increase in entropy
| Sample | [X] | [Y] | [Z] |
|---|---|---|---|
| 1 | 0.5 | 2 | 10 |
| 2 | 4 | 0.25 | 10 |
| 3 | 4 | 1 | 20 |
| 4 | 1 | 1 | 5 |
a) Sample 1
b) Sample 2
c) Samples 1 and 2
d) Samples 1, 2, and 4
b) Sample 2
c) Samples 1 and 2
d) Samples 1, 2, and 4
a) 1
b) 2
c) 4
d) 10
b) 2
c) 4
d) 10
| Compound | NO2 | N2O4 |
|---|---|---|
| Initial pressure (atm) | 10 | 0 |
| Final Pressure (atm) | 2.0 | 4.0 |
|
Related Links: Chemistry Chemistry Quizzes AP Chemistry Notes Reversible Reactions |
To link to this Equilibrium Problems page, copy the following code to your site:
