Enduring Understanding 5.B: Energy Transfer and Phase Transitions
- Energy can be transferred between two systems either
- As heat
- Through one system doing work on the other system.
- Work is all forms of energy transfer that is not heat transfer. For example, it can be mechanical (e.g. lifting a weight), electrical (causing current to flow), pressure-volume (changes in volume of a gas)...
- In AP Chemistry, work calculations are limited to pressure-volume changes.
- When energy is transferred from one system to another:
- The energy transferred from system 1 is equal in magnitude to that absorbed by system 2.
- In other words, energy cannot be created or destroyed, only transferred
- This is called 'conservation of energy'.
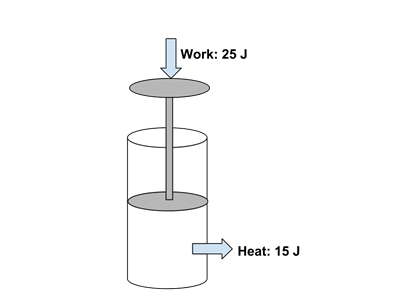
- Example: What is the energy change in a piston system that has 25 joules of work done on it, and loses 15 joules of heat to its surroundings?
- The piston has gained 25 J energy and lost 15 J, so the net energy change in the system is +10 J.
- Chemical systems under go three types of processes that change their energy.
- Heating/cooling (like liquid water warming from 10 °C to 50 °C)
- Phase changes (ice melting to water at 0 °C, water boiling to steam at 100 °C)
- Chemical reactions.
- Phase transitions involve the absorption or release of energy by the system, with no change in temperature.
- When a liquid boils, energy is absorbed for the liquid to gas phase transition. The amount of energy required to vaporize one mole of a substance is the molar enthalpy of vaporization. The amount of energy required to vaporize a given mass m of a substance is:
ΔH = (m)(Δ Hvaporization) - When a solid melts ('fusion'), energy is absorbed for the solid to liquid phase transition. The amount of energy required to melt one mole of a substance is the molar enthalpy of fusion. The amount of energy required to melt a given mass m of a substance is:
ΔH = (m)(Δ Hfusion) - The amount of energy absorbed when a substance boils, and released when the same amount of substance condenses, is the same. Similarly, the amount of energy absorbed when a substance melts, and released when the same amount of substance freezes, is the same.
- Sublimation, a substance going straight from solid to gas phase, also involves the absorbing of energy.
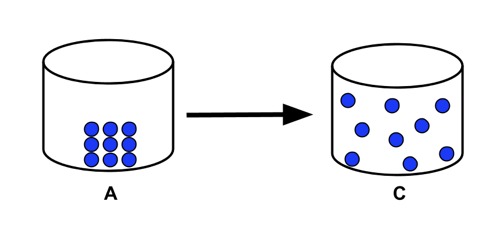
- Sample Problem 1: Consider the phase transition illustrated by the particle diagram below. Would this transition involve the system absorbing or releasing energy?
- The transition illustrated is a solid A going to a gas C, or sublimation. This would involve the absorption of energy from the surroundings.
- Sample Problem 2: How much energy would be absorbed or released if 100 g of ice at 0 °C were converted to steam at 100 °C? Use the following values.
- molar enthalpy of fusion, ΔHfusion = 334 J/g
- molar enthalpy of vaporization, ΔHvaporization = 2200 J/g
- molar heat capacity of water, Cp = 4.2 J/g.°C
- The phase transition is solid to gas, so energy will be absorbed.
- The process involves the ice melting to water, the water heating from 0 °C to 100 °C, then the water boiling to steam.
- The energy absorbed will be the heat of fusion + the change in temperature of the liquid + the heat of vaporization, qfusion + qheating + qvaporization
- qfusion = 100 g x 334 J/g = 33400 J
- qheating = 100 g x 4.2 J/g•°C x 100 °C = 42000 J
- qvaporization = 100 g x 2200 J/g = 220000 J
- qfusion + qheating + qvaporization = 33400 + 42000 + 220000 = 295400 J, or 295 kJ absorbed.
To link to this Energy Transfer and Phase Transitions page, copy the following code to your site:
