Enduring Understanding 2.C.1: Covalent Bonding
- The strong electrostatic forces holding atoms together in units (molecules, unit cells in crystals) are called chemical bonds.
- Covalent bonds involve the sharing of pairs of valence electrons between two or more atoms in a molecule.
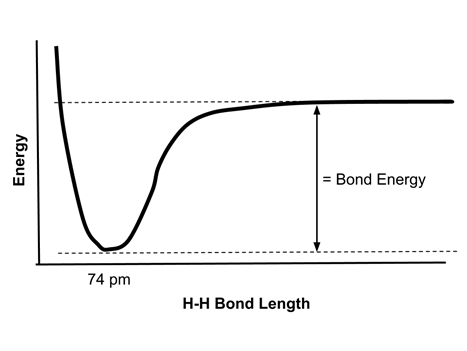
- Consider the covalent bond of hydrogen, H2. The energy of two hydrogen atoms at various distances from each other can be displayed graphically: <
- The bond energy of the H-H bond is the difference between the minimum energy and the energy of the two atoms at a large distance from each other. As two atoms approach each other, the two electrons are increasingly attracted to both nuclei and the potential energy drops.
- The energy is at a minimum at a distance of 74 pm. This is the bond length of the H2 molecule.
- At bond distances of less than 74 pm, repulsion between the positively charged nuclei increases, so the energy increases rapidly.
- This electron sharing can be unequal, depending on the relative electronegativities of the two atoms. Electronegativity is the relative tendency of an atom to attract electrons to it.
- The electrons will be concentrated near the more electronegative atom, resulting in the molecule having a dipole.
- Electronegativity displays periodic trends, similar to ionization energy. It increases going up and across the periodic table. F is the most electronegative atom, and Cs is one of the least electronegative atoms.
- Bonds between atoms with the same or similar electronegativity are considered nonpolar. Note, in particular, that bonds between carbon and hydrogen are considered nonpolar.
- As electronegativity differences become greater, the bond can become more ionic in character.
- Bonds between metals and nonmetals tend to be ionic.
- Bonds between nonmetals are more covalent.
To link to this Covalent Bonding page, copy the following code to your site:
