Enduring Understanding 6.C.2: Buffers
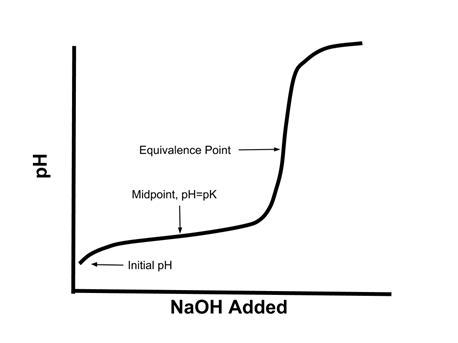
- Consider again the titration curve of a weak acid:
- Notice how in the middle of the curve, especially around the midpoint, there is a relatively small change in pH when a given amount of strong base is added.
- This is an illustration of a property of buffers - systems where the addition of small amounts of acid or base do not greatly change the pH of the system
- A buffer contains significant concentrations of a conjugate acid-base pair. When strong acid or base is added, they react with either the weak acid or the weak base, and the pH does not change much.
- Weak acids and their conjugate bases, or weak bases and their conjugate acids make good buffers.
- Examples include acetic acid/acetate, pyridinium/pyridine, carbonic acid/hydrogen carbonate...
- Buffers are best able to resist changes in pH when there is a near equal concentration of the conjugate acid and base (i.e. when the pH = pKa of the acid/base pair). Example: To create a buffer with a pH of 5.5, which of the following acid/base pairs would we choose? HCOOH/HCOONa (pKa = 3.7); C5H5N/C5H5NHCl (pKa = 5.2); HClO/NaOCl (pKa = 7.5).
- Pyridine/pyridinium chloride would form the best pH = 5.5 buffer, because its pKa (5.2) is closest to 5.5.
- A buffer can only resist changes in pH as long as some of each of the acid and base remain. If they are consumed (for example, by the addition of a large amount of strong acid or base) the solution is no longer a buffer.
- Sample Question: A 1L buffer solution of 0.1M acetic acid and 0.1M sodium acetate will have its buffering capacity destroyed by the addition of which of the following?
- Answer: (d), 0.25 mol hydrochloric acid.
- As long as there is some acetic acid and sodium acetate present, the system is a buffer, so (a) and (b) will have no effect. (c), sodium hydroxide, will consume some of the acetic acid, but there is not enough to consume all the acetic acid (0.1 mol) present. However, 0.25 mol of HCl is enough to consume all the sodium acetate (0.1 mol) present, so this will remove the buffering capacity of the solution.
a) 0.2 mol sodium acetate
b) 0.3 mol acetic acid
c) 0.05 mol sodium hydroxide
d) 0.25 mol hydrochloric acid
b) 0.3 mol acetic acid
c) 0.05 mol sodium hydroxide
d) 0.25 mol hydrochloric acid
To link to this Buffers page, copy the following code to your site:
