Enduring Understanding 5.C.1: Bond lengths and dissociation energies
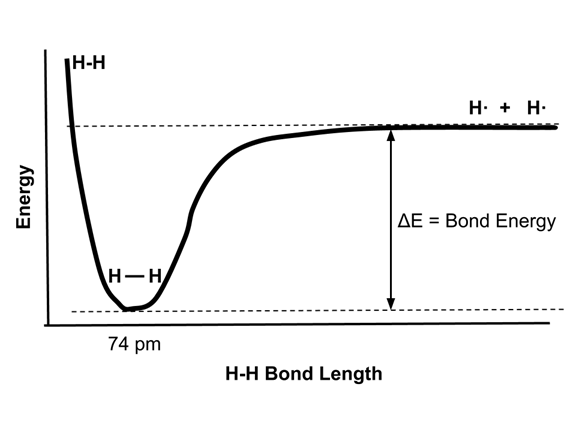
- As two atoms approach each other, the electrons of one atom are attracted to the nucleus of the other atom. The potential energy of the system drops.
- When the two atoms get very close, the electron clouds repel each other and the energy of the system increases rapidly.
- The distance at which the energy is minimized is called the bond length. The energy drop of the system from the two separated atoms is the bond energy.
- The bond length and bond energy are illustrated in the following energy diagram of the hydrogen molecule, H2.
- The bond dissociation energy, ΔE, is the energy released by two isolated hydrogen atoms when they come together to the energy minimum bond distance of 74 pm. This energy is 436 kJ/mol for H2.
- Bond making and bond breaking are opposing processes that involve the same amount of energy. Therefore, the formation of 1 mole of H-H bonds from 2 moles of H atoms releases 436 kJ/mol, and the breaking of 1 mole of H-H bond to form 2 moles of H atoms requires 436 kJ/mol.
- Some typical bond dissociation energies are illustrated here.
- Shorter bonds tend to be stronger than longer bonds.
- Multiple (double) bonds are stronger than single bonds because they involve the sharing of more electron pairs.
- Sample question: Which compound would have a stronger (higher bond dissociation energy) carbon-nitrogen bond, CH3NH2 or CH3CN?
- Acetonitrile, CH3CN, has a triple carbon-nitrogen bond, which would be much stronger and have a higher dissociation energy than methylamine, CH3NH2, which only has a single C-N bond.
| Bond | Bond Dissociation Energy (kJ/mol) | Notes |
|---|---|---|
| Cl-Cl | 242 | Chlorine, shorter than Br2 or I2 |
| Br-Br | 192 | |
| I-I | 151 | Iodine, very long & weak bond |
| C-C | 356 | Alkane, as in ethane |
| C=C | 632 | Alkene, as in ethylene, double bond |
| C≅C | 837 | Alkyne, as in acetylene, triple bond |
| N≅N | 945 | Dinitrogen, one of the strongest bonds known |
To link to this Bond lengths and dissociation energies page, copy the following code to your site:
