Enduring Understanding 3.A.1: Balanced Chemical Equations
- Chemical equations are used to describe the rearrangement of atoms and electrons during a chemical reaction.
- Because atoms can be neither created nor destroyed, and always react in fixed proportions, each side of the equation must contain the same number of each kind of atom. This is called a balanced equation.
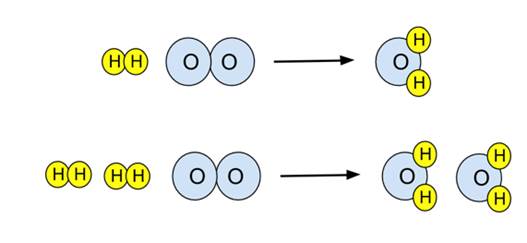
- Example: For the reaction of hydrogen and oxygen to give water,
- This equation is unbalanced. There are two oxygens on the reactant side, only one on the product side. To balance it, the number of product water molecules must be increased to 2, and then the number of reactant H2 molecules must also be increased to 2. This provides the balanced equation:
- This reaction can be illustrated in a particulate diagram. The unbalanced equation on top, the balanced equation below.
- Note the upper diagram is not balanced - there are a different number of oxygen atoms on either side of the arrow. The lower equation is balanced.
H2 + O2 → H2O
2H2 + O2 → 2H2O
To link to this Balanced Chemical Equations page, copy the following code to your site:
