Zinc Nitrate Formula
Zinc nitrate, also known as zinc dinitrate, is an inorganic chemical compound used as precursor and catalyst in many organic synthesis.
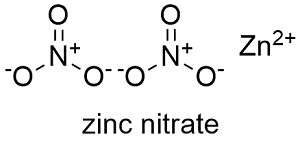
Formula and structure: The zinc nitrate chemical formula is Zn(NO3)2 and the molar mass is 189.36 g mol-1 in its anhydrous form and 297.49 g mol-1 when it is in its hexahydrate form. It is formed by the nitrate anion NO32- and the Zinc2+ cation. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Zinc nitrate can be found in nature in some rocks and geological formations.
Preparation: Zinc nitrate can be prepared from the reaction of nitric acid on zinc or zinc oxide.
Zn + 2 HNO3 (diluted) → Zn(NO3)2 + H2
Or 4 Zn + 10 HNO3 (concentrated) → 4 Zn(NO3)2 + NH4NO3 + 3 H2O if the acid is concentrated.
Physical properties: Zinc nitrate is a colorless, deliquescent and crystalline solid. Its density is 2.065 g mL-1. Its melting and boiling point are 110 °C and 125 °C respectively, when it is the hexahydrate form, the melting point is 35 ºC. Zinc nitrate is soluble in water and ethanol.
Chemical properties: Zinc nitrate is dissociated in aqueous solution according to the next reaction:
Zn(NO3)2 → Zn2+ + 2 NO3-
This reaction is highly favored by the system and it explains the high solubility of this salt in water.
Uses: Zinc nitrate is mostly used as catalyst in synthetic organic reactions where the metallic zinc is required. It can also be used in the production of some agricultural products and to oxidize metals.
Health effects / safety hazards: Zinc nitrate is harmful if swallowed and cause skin irritation and eye damage. It is not combustible but accelerates the burning of combustible materials.
|
Related Links: |
