Tricalcium phosphate Formula - Tricalcium Phosphate Uses, Properties, Structure and Formula
Tricalcium phosphate is one of the most common and important members of the calcium phosphate family of minerals, which are made of calcium cations with different phosphate anions such as orthophosphates, metaphosphates or pyrophosphates. It is also called as simply calcium phosphate.
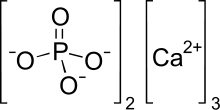
Formula and structure: The chemical formula of tricalcium phosphate is Ca3(PO4)2. Its molecular formula is Ca3O8P2 and its molar mass (molecular weight) is 310.177 g/mol. It is an ionic compound composed of three bivalent calcium cations (Ca2+) and two phosphate anions (PO43-), in which phosphorous atom is attached to one oxygen atom through a double bond, and to three other oxygen atoms through single bonds.
Occurrence: Tricalcium phosphate is found in cow milk and blood. Calcium phosphate is also the major component of bones and tooth enamel, as the mineral hydroxyapatite.
Preparation: Ca3(PO4)2 is prepared industrially by reacting aqueous solutions of calcium chloride and sodium triphosphate with excess ammonia. Pure tricalcium phosphate is also obtained by the high temperature calcination of hydroxyapatite mineral Ca5(PO4) 3OH.
Physical properties: Tricalcium phosphate is a white, crystalline powder that is odorless and tasteless. It has a density of 3.14 g/mL, and a melting point of 1670 °C.
Chemical properties: It is moderately soluble in water, and readily dissolves in dilute hydrochloric acid and nitric acid. It is insoluble in alcohols and acetic acid. Tricalcium phosphate is unusual in that its water solubility decreases at higher temperatures, causing precipitation. It is a stable compound which is not very chemically reactive.
Uses: Tricalcium phosphate has several applications in the manufacture of fertilizers, phosphoric acid and other phosphorous compounds, porcelains, pottery, textiles, luminescent materials and dental powders. It is also a food additive and is used as a nutritional supplement, baking agent, meat tenderizer, for clarifying syrups, and as a buffer in food. It also has applications as an antacid and pharmaceutical filler in tablets.
Health effects/safety hazards: Tricalcium phosphate is not considered toxic or irritating unless swallowed in very high concentrations. It is considered safe as a food additive in recommended limits.
|
Related Links: |
