Tartaric acid Formula
Tartaric acid is an important carboxylic acid, which is also called as dihydroxy butanedioic acid.
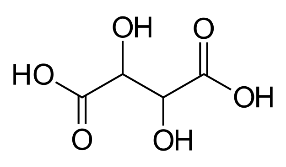
Formula and structure: The chemical formula of tartaric acid can be written as HO2C-CH(OH)-CH(OH)-CO2H or (CH(OH)COOH)2. Its molecular formula is C4H6O6 and its molar mass is 150.09 g/mol. It is a dihydroxy (having two OH groups) and dicarboxylic (having two COOH groups) acid. The simple chemical formula of tartaric acid is shown below.
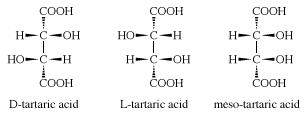
Tartaric acid is a chiral molecule which exists as three stereoisomers (having same composition but different orientations in space): D-tartaric acid, L-tartaric acid and meso-tartaric acid, which have slightly different structures as shown below. Racemic tartaric acid is an equal mixture of D- and L-tartaric acid.
Occurrence: Tartaric acid is found in several plants, fruits and vegetables including tamarinds, bananas and grapes. Several of its salts occur during the fermentation of grapes to form wine.
Preparation: The L-(+)-tartaric acid isomer is the most common form, and thus is produced in the largest quantities. It is prepared by reacting calcium tartrate with aqueous sulfuric acid to give L-(+)-tartaric acid:
Ca(O2CCH(OH)CH(OH)CO2) + H2SO4 → HO2CCH(OH)CH(OH)CO2H + CaSO4
Physical properties: Tartaric acid is a white crystalline solid with a density of 1.79 g/mL and melting point of 206°C.
Chemical properties: Tartaric acid is an alpha-hydroxy carboxylic acid, and is diprotic. All the forms of tartaric acid are readily soluble in water. It can form salts with bases, and some of the important salts of tartaric acid are cream of tartar (potassium hydrogen tartrate) and Rochelle salt (potassium sodium tartrate). Tartaric acid is an optically active compound, and was one of the first chemicals to be studied regarding stereoisomerism.
Uses: Tartaric acid has many applications in the food industry due to its sour taste and antioxidant properties. It is widely used in carbonated drinks, effervescent tablets, sour candies, gelatins, jellies, baking powders, and taffies. It also has many industrial uses such as in photographic printing and development, polishing metals, wool dyeing, etc. The tartrate salts are used in silvering mirrors, cleaning brass, dyeing mordant, and as insecticide.
Health effects/safety hazards: Tartaric acid is safe for human consumption is low doses and is used in food products. However, in large doses it can be harmful. It is a muscle toxin, which can cause paralysis and death when swallowed in large amounts.
|
Related Links: |
Related Topics
Acid Names Formulas
Chemistry Formulas
Formulas: Physics Formulas and Math Formulas
General Chemistry topics
