Sulfur Trioxide Formula
Sulfur trioxide, also written as sulphur trioxide, is an inorganic compound largely used as a precursor in the preparation of sulfuric acid.
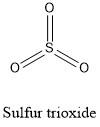
Formula and structure: Sulfur trioxide chemical formula is SO3 and the molar mass is 80.01 g mol-1. The structure of this oxide is formed by one cation hexavalent Sulfur6+ and three anions O2-. The geometry of the molecule is trigonal planar, with each atom of oxygen bond to the centered sulfur cation and forming an angle of 120 °C between each oxygen atom. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Sulfur trioxide is not found in nature.
Preparation: Sulfur trioxide can be synthesized in laboratory through some methods. The most used is the oxidation of sulfur dioxide using as catalysts vanadium pentoxide activdate at 600 °C. Other method is the dehydratation and cracking of the sodium bisulfate.
2 NaHSO4 → Na2S2O7 + H2O (Dehydration)
Na2S2O7 → Na2SO4 + SO3 (Cracking)
Physical properties: Sulfur trioxide is a colorless or white crystalline solid or a colorless liquid. In contact with air, it can emit fume. Its density is 1.92 g mL-1. Sulfur trioxide melting point is 16.9 °C and boiling point is 45 °C, so it can be a solid or a liquid at room temperature. In contact with water, it reacts violently to form sulfuric acid.
Chemical properties: Sulfur trioxide is a Lewis acid, meaning it can accept electrons from a donor and has empty orbital that allow it. Sulfur trioxide can then react with some Lewis bases that can act as the donor of the electrons. Such bases, as trimethylamine and pyridine, form complex with sulfur trioxide.
Sulfur trioxide can be used to produce sulfuric acid through the addition of water. However, this reaction can be reversible and the temperature determines the direction of the reaction, being 340 °C the equilibrium temperature in which all the three components (water, sulfuric acid and sulfur trioxide) coexist:
SO3 (g) + H2O (l) → H2SO4 (aq)
Uses: Sulfur trioxide is largely used in the obtention of sulfuric acid in which water reacts with the trioxide to form the acid. It can also be used in other sulfonation reactions that are very useful in the obtention of precursors in the chemical and pharmaceutical industries.
Health effects / safety hazards: Sulfur trioxide can cause severe damage in lungs by inhalation. It is very toxic by ingestion. Sulfur trioxide is highly corrosive and reacts violently with water to produce the sulfuric acid. It can oxide some compounds and also acts as a dehydrating agent.
|
Related Links: |
