Sodium hypochlorite Formula - Sodium hypochlorite Uses, Properties, Structure and Formula
Sodium hypochlorite, also called as liquid bleach, is a powerful oxidizing agent that is widely used as a disinfecting and bleaching agent.
Formula and structure: The chemical formula of sodium hypochlorite is NaClO, and its molar mass is 74.44 g/mol.
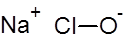
It is an ionic compound composed of the sodium metal cation (Na+) bonded to the hypochlorite anion (ClO-).
Preparation: It is prepared industrially by the Hooker process, in which dilute sodium hydroxide is reacted with chlorine gas, to produce NaClO, along with sodium chloride and water.
2 NaOH + Cl2 → NaClO + NaCl + H2O
Physical properties: In the pure state, it exists as an unstable light green solid, with a density of 1.11 g/mL, a melting point of 18 °C, and a boiling point of 101 °C. It is more commonly found as an aqueous pale greenish or yellow solution. The aqueous NaClO solutions are available in several different concentrations or strength (from 1% to 25%), depending on its end uses.
Chemical properties: Sodium hypochlorite is a good oxidizing agent. It reacts with protic acids such as HCl, to form salts while releasing toxic chlorine gas. It also reacts with some acids to form hypochlorous acid (HClO). In water, it decomposes into sodium and chloride ions, as well as the powerful oxidizing agent, hydroxyl radical (OH.). NaClO also decomposes into sodium chloride and oxygen.
Uses: Its main uses are as a bleaching agent and disinfectant for both household and industrial purposes. It is also used in water treatment plants, swimming pools, in some medical and dental treatments, in the food industry as a disinfecting agent, in homes as a deodorizing and cleaning agent, and in laundry detergents as a stain remover and bleach.
Health effects/safety hazards: Sodium hypochlorite is a toxic and corrosive compound at higher concentrations. When swallowed, it can be very toxic. It also reacts with some acids as well as ammonia to produce toxic gases such as chlorine, which can lead to severe eye irritation and respiratory problems. Strong solutions of bleach can also cause skin burns.
|
Related Links: |
