Sodium bicarbonate Formula
Sodium bicarbonates, also called sodium hydrogen carbonate, is a inorganic salt widely used in the food, pharmaceuthical industries. It is also used as household chemical.
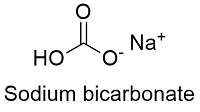
Formula and structure: The sodium bicarbonate chemical formula is NaHCO3 and its molar mass is 84.006 g mol-1. The molecule is formed by the sodium cation Na+ and the bicarbonate anion HCO3-. The structure of the sodium bicarbonate lattice is monoclinic. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Sodium bicarbonate is found in deposits around the world. The deposits of this salt are called nahcolite.
Preparation: Although sodium bicarbonate can be found in nature, it is mostly obtained from synthetic methods. The Solvay process is the most used, in this process the sodium chloride, ammonia and carbon dioxide react to yield sodium carbonate, which react with carbon dioxide and water to produce sodium bicarbonate.
NaCl + NH3 + CO2 + H2O → Na2CO3 + NH4Cl
Na2CO3 + CO2 + H2O → 2 NaHCO3
Physical properties: Sodium bicarbonate is an odorless, white crystalline solid or fine powder. It has a slightly alkaline taste. Its density is 2.20 g mL-1 and it decomposes in temperatures above 50 ºC. The decomposition yields to sodium carbonate. It is highly soluble in water and poorly soluble in acetone and methanol. It is insoluble in ethanol.
Chemical properties: Sodium bicarbonate is an amphoteric compounds, it means the compound has a character acids an basic at the same time. It is highly soluble in water, resulting in a slighty alkaline solution, which is very used to prepared akalis solutions:
HCO3- + H2O → H2CO3 + OH-
The reactions in acids solutions results in sodium salt and carbonic acid (reaction I), while in basic solutions are formed carbonates and water (reaction II):
NaHCO3 + HCl → NaCl + H2CO3 (I)
H2CO3 → H2O + CO2(g)
NaHCO3 + NaOH → Na2CO3 + H2O (II)
Other important reaction of sodium bicarbonate is the decomposition in temperatures higher than 50 ºC, that produces sodium carbonate, water and carbon dioxide.
2 NaHCO3 → Na2CO3 + H2O + CO2
Uses: Sodium bicarbonate is largely used in food industry; it is especially sold as the main component of baking powder. It is also an additive in flours and other foods and beverages. Sodium bicarbonates is used to prepare alkali solution (pH higher than 8). Moreover, it is used in pharmaceutical, to produce antacids and in soaps and detergents, deodorants, toothpastes and other industries. It is also apply in fire extinguishers.
Health effects / safety hazards: Sodium bicarbonate can cause serious eye damage. It is not flammable but in contact with acid can produce non-toxic fumes of carbon dioxide.
|
Related Links: |
