Sodium acetate Formula
Sodium acetate, also known as sodium ethanoate (abbreviated as NaOAc), is a sodium salt of acetic acid, which is largely use in food, textile, pharmaceutical and chemical industries.
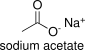
Formula and structure: The chemical formula of sodium acetate is CH3COONa. Its molecular formula is C2H3NaO2 and its molar mass is 82.03 g mol-1. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Sodium acetate occurs naturally in plants and animals tissues. This salt can be found in either, the anhydrous or trihydrated form. Both of the ion, Na+ and CH3COO- are present in the organisms and exerting vital roles: sodium ion as regulator of total body water and acetate ion as hydrogen acceptor.
Preparation: Sodium acetate can be prepared through a very simple and cheap reaction consisting in the neutralization of acetic acid. The synthesis uses vinegar (acetic acid 5-8%) or glacial acetic acid, washing soda (sodium carbonate) or baking soda (sodium bicarbonate) or sodium hydroxide. When the reaction includes sodium carbonate or bicarbonates, it produces carbonic acid and sodium acetate. Carbonic acid readily decomposes under normal conditions into carbon dioxide and water. If the reaction is prepared with sodium hydroxide, sodium acetate and water will be the only products.
CH3COOH + NaHCO3 → CH3COONa + H2CO3
H2CO3 → CO2 + H2O
CH3COOH + NaOH → CH3COONa + H2O
Physical properties: Sodium acetate is a hygroscopic white crystalline powder with vinager (acetic acid) odor. Its melting point is 324 ºC. Its density is 1.5 g mL-1. Sodium acetate is higly soluble in water (the solubility is 46.5 g sodium acetate in 100 mL of water).
Chemical properties: Sodium acetate is the conjugated base of acetic acid, thus a solution of acetic acid/sodium acetate can be used to prepare buffer solutions, in order to control pH. Sodium acetate solution in water is weak alkaline. When heat is above 324 ºC, this salt decomposes, producing acetic acid fumes.
Uses: Sodium acetate is widely use by the food, pharmaceutical and chemical industries. In food industries, sodium acetate is added as a seasoning due its vinegar taste. For example, in the fries potatoes chips, it is used as a salt and vinegar flavor. Sodium acetate solution is used as medicine alternative to sodium chloride to provide sodium ion (Na+) to the organism.
As mentioned in the chemical properties, sodium acetate is used to buffering solutions, especially to biochemical and pharmaceutical application. Moreover, sodium acetate is used in neutralizationof sulfuric acid waste in the textile industry.
Health effects/safety hazards: Sodium acetate is stable. It is incompatible with strong oxidizing agents. This salt is mildly irritating of eyes, skin and respiratory tract.
|
Related Links: |
Related Topics
Ionic and Net Ionic Equations
