Silver chloride Formula - Silver chloride Uses, Properties, Structure and Formula
Silver chloride is an important photosensitive inorganic material widely used in photographic applications. It is also called as silver(I) chloride.
Formula and structure: The chemical formula of silver chloride is AgCl, and its molar mass is 143.32 g/mol. Silver chloride is a simple ionic compound consisting of the silver cation (Ag+) and chloride anion (Cl-). In the solid state, AgCl adopts a crystalline structure similar to that of sodium chloride (NaCl), with each silver cation being surrounded by six chloride anions in an octahedral geometry.
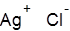
Occurrence: Silver chloride is found in nature as the mineral chlorargyrite. However, chemical synthesis is the main and inexpensive method to obtain AgCl.
Preparation: Silver chloride is industrially produced by the simple reaction between aqueous solutions of silver nitrate (AgNO3) and sodium chloride (NaCl), resulting in a white AgCl precipitate which is easily filtered off and collected.
AgNO3 + NaCl → AgCl + NaNO3
Physical properties: Silver chloride exists as a white crystalline solid with a density of 5.56 g/mL, a melting point of 455 °C and a boiling point of 1,547 °C. It is insoluble in water despite being an ionic compound.
Chemical properties: Silver chloride is insoluble in water, alcohol and dilute acids but soluble in ammonia and concentrated acids. It is a photosensitive material (it undergoes chemical reactions in the presence of light), and upon illumination, it decomposes into silver metal and elemental chlorine. This reaction is characterized by the darkening of the AgCl sample, and makes AgCl an important chemical for photographic applications.
2 AgCl → 2 Ag + Cl2
Uses: Many of the applications of AgCl are based on its light sensitive conversion to metallic silver, such as preparation of photographic films and photochromic lenses. It is an important reference electrode used in cells, and is also used to prepare infrared windows, pottery glazes, and stained glass. AgCl has disinfectant/antiseptic properties and is used in antimicrobial products, wound healing products, personal deodorants, water treatment, and antidotes for mercury poisoning.
Health effects/safety hazards: Silver chloride is not toxic at low concentrations and is used in medical and disinfecting applications. However, if swallowed or inhaled in high concentrations, it may cause irritation of mucous membranes, greyish discoloration of the internal tissues (argyria) and kidney damage. Skin or eye contact with silver chloride can cause greyish discoloration of the skin and tissues.
|
Related Links: |
