Silane Formula
Silane is an inorganic gas compound which has particular interest as precursor in the production of silicon.
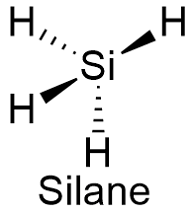
Formula and structure: The silane chemical formula is SiH4 and its molar mass is 32.12 g mol-1. The molecule is formed by four hydrogen atoms join to an central silicon atom. The silane structure is highly similar to the methane structure, a tetrahedral structure with a central atom and four substituent forming bonds with an angle of 109º. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Silane is not found freely in nature, it should be produced by chemical methods. However, it is possible some hydride of silicon can react in nature to form silane.
Preparation: Silane is produced from magnesium silicide and hydrochloric acid:
Mg2Si + 4 HCl → 2 MgCl2 + SiH4
It can also be synthesized by the reactions between silicon, hydrogen and silicon tetrachloride:
Si + 2 H2 + 3 SiCl4 → SiH4 + SiH2Cl2
A third route includes the reduction of silicon dioxide (SiO2) in presence of aluminium and hydrogen:
3 SiO2 + 6 H2 + 4 Al → 3 SiH4 + 2 Al2O3
Physical properties: Silane is a colorless with repulsive odor gas. Its density is 1.114 g mL1- and its melting and boiling points are -185 ºC and -112 ºC, respectively. Silane is poorly soluble in water.
Chemical properties: Silane is formed by 4 bonds silicon-hydrogen, these bonds show a low polarity, and thus silane may form many complexes with metals as iron, copper and manganese. These bonds Si-H are also weak, so silane can be easily decomposes by environmental factors such as temperature and it suffer spontaneous combustion in air.
Uses: Silane is used by the pharmaceutical and chemical industries in large scale and while the medical applications include dentistry utensils, the chemical applications include raw material to produce polymers, repellents, carbon fibers and glass. Silane is also used to manufacture combustibles and solar photovoltaic module.
Health effects / safety hazards: Silane is poisonous by inhalation. It is an eyes and skin irritant. It is highly flammable and easily ignites in air and it is also a potent combustion agent. Silane also reacts with oxidizing agents.
|
Related Links: |
