p-Toluenesulfonyl chloride Formula
p-Toluenesulfonyil chloride, also known as tosyl chloride or TsCl or TosCl, is an organic compound largely used in chemical synthesis as a protective group.
Formula and structure: The tosyl chloride chemical formula is C7H7ClO2S and its molar mass is 190.15 g mol-1. The molecule is formed by one toluene core, which has in para position a sulfonyl chloride. The structure is completely planar, due to most of the atoms need a sp2 hybridization to form the double bonds. Its chemical structure can be written as below, in the common representations used for organic molecules.
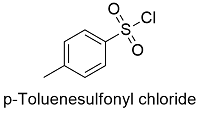
Occurrence: Tosyl chloride is not formed naturally, it is produced just by organic synthesis.
Preparation: Tosyl chloride is largely produced through the treatment of toluene with chlorosulfonic acid, in a reaction knows as chlorosulfonation:
CH3C6H5 + SO2Cl2 → CH3C6H4SO2Cl + HCl
Physical properties: p-Toluenesulfonyil chloride is a white to gray powder or solid with a characteristic odor. The p-Toluenesulfonyil chloride melting point is 65-67 ºC and its boiling point is 134 ºC. It is insoluble and denser than water.
Chemical properties: p-Toluenesulfonyil chloride is a is a strong electrophile due to the deficit of electronic charge on the sulfur atom. This deficit is associated to the bonds with oxygen atoms, which are more electronegative causing the dislocation of the electrons to them. Thus, the TosCl can be attack by nucleophiles, such as alcohol to promote reaction of nucleophilic substitution, where the chlorine atom is replaced. The most important function of the Tosyl chloride is being a protecting group in organic synthesis.
Uses: p-Toluenesulfonyil chloride is used in chemical industry and chemical laboratories as a protection agent (avoiding a side-reaction over some group on the molecule). It is used to produce ester from alcohol via a substitution. It can also be used to prepare sulfonamides from amines, which has a large value in pharmaceutical industry:
CH3C6H4SO2Cl + R2NH → CH3C6H4SO2NR2 + HCl
Health effects / safety hazards: p-Toluenesulfonyil chloride cause severe skin burns and eyes damage. It is also an allergic agent and is harmul to the environment. It is not flammable but it is combustible. When heated produces toxic fumes.
|
Related Links: |
