Potassium Sulfide Formula
Potassium sulfide, is an inorganic salt found as a mixture with potassium hydrosulfide and used as this mixture to manufacture pyrotechnics.
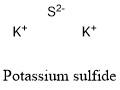
Formula and structure: The potassium sulfide chemical formula is K2S. The molar mass is 110.26 g/mol. The molecule is formed by two potassium cation K+ and one sulfide anions S2-. The two ions are bound trough ionic bond. The geometry of the molecule is an antifluorite structure, with a cation K+ being surrounded by eight sulfide anions. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Potassium sulfide is not found in nature.
Preparation: Potassium sulfide can be prepared through the reaction of pure carbon with potassium sulfate at high temperatures:
K2SO4 + 4 C → K2S + 4 CO
As a by-reaction, it is formed carbon monoxide.
Physical properties: Potassium sulfide is a colorless crystal with a typical sulfur smell. The density of this salt is 1.74 g/mL. Its melting point is 840 °C and the boiling point is 912 °C. Potassium sulfide reacts with water (see chemical properties) but it is soluble in ethanol and glycerol. It is insoluble in ether.
Chemical properties: Potassium sulfide suffer spontaneous hydrolysis in water, reacting to form potassium hydroxide and potassium hydrosulfide:
K2S + H2O → KOH + KSH
Potassium sulfide is not found as an isolated compound, it is always found as forming the mixture with KSH.
Uses: Potassium sulfide is used as a component of pyrotechnics. It is particularly used in Asia to manufacture the fireworks senki hanabi. Potassium sulfide is also used in the production of glitter.
Health effects / safety hazards: Potassium sulfide is dangerous for health. The toxic fumes formed after heating are harmful for inhalation. It is highly flammable and its particles can form explosives mixtures in air, igniting spontaneously.
|
Related Links: |
