Potassium phosphate Formula
Potassium phosphate, also known as potassium phosphate monobasic or potassium bisphosphate is a salt used as fertilizer and food additive.
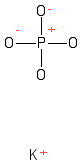
Formula and structure: Although there are several potassium phosphate salts that vary according to the arrangement and number of ions of each one, in this article will be consider the potassium phosphate monobasic. The potassium phosphate monobasic chemical formula is KH2PO4. The molar mass is 136.086 g/mol. This molecule is formed by one cation K+ and one biphosphate anion H2PO4-. The structure varies with the temperature; at room temperature the structure is orthorhombic, while at high temperature it is monoclinic. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Potassium phosphate can be found in the living organisms as being part of some biochemical pathways and it can also be found in ores and minerals.
Preparation: Potassium phosphate is produced through the reaction of phosphoric acid and potassium carbonate, through the reaction:
H3PO4 + K3CO2 → KH2PO4 + CO2
It can also be produced by using potassium hydroxide or chloride instead of potassium carbonate.
Physical properties: Potassium phosphate is a white, odorless and deliquescent powder. The density of this salt is 2.33 g/L. Its melting point is 252.6 °C and the boiling point is 400 °C. Potassium phosphate is highly soluble in hot water, although it is also soluble in cold water but the rate is smaller. It is also slightly soluble in ethanol.
Chemical properties: Potassium phosphate exhibits interesting electric properties. The compound is ferroelectric below -150 °C, meaning that the molecule has a spontaneous electric polarization that can be used in order to get conductors and piezoelectric materials.
Uses: Potassium phosphate is used in the laboratories, pharmaceutical and food industries as buffering agents. The buffers prepared with potassium phosphate have the advantage of reaching the physiological pH 7.4. It is also used as emulsifying salt for manufacturing of cheese and meat. Potassium phosphate is also used as a fertilizer and due to its chemical properties can be used in the production of piezoelectric materials.
Health effects / safety hazards: Potassium phosphate is dangerous for ingestion in large quantities. It is not combustible, although when heated, can emit toxic fumes.
|
Related Links: |
