Potassium Permanganate Formula
Potassium permanganate is an inorganic compound used as medicine to treat dermatitis. It is on the World Health Organization (WHO) model list of essential medicines needed in a basic health system.
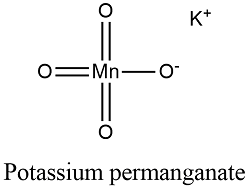
Formula and structure: The potassium permanganate chemical formula is KMnO4 and its molar mass is 158.034 g mol-1. The molecule is formed by the potassium cation K+ and the permanganate anion MnO4-. The structure of the potassium permanganate lattice is orthorhombic. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Potassium permanganate is not found like a free chemical compound in nature. It is prepared from other minerals that contain manganese such manganese (IV) oxide.
Preparation: Potassium permanganate can be produced from the mineral pyrolusite, which contain manganese dioxide (MnO2). This dioxide is oxidized with potassium chlorate or potassium hydroxide. This reaction is completed with chlorine, air or carbon dioxide and it is usually heated to 200-350 ºC:
2 MnO2 + 4 KOH + O2 → 2 K2MnO4 + 2 H2O
The oxidation of the MnO2 can also be obtained through an alkaline electrolytic cell:
2 K2MnO4 + 2 H2O → 2 KMnO4 + 2 KOH + H2
Physical properties: Potassium permanganate is an odorless, purple to magenta crystalline solid or needles. Its density is 2.703 g mL-1. The melting point is 240 ºC and it decomposes at temperature above 250 ºC. It is slightly soluble in water and soluble in acetone, acetic acid, pyridine and methanol. It decomposes in ethanol and organic solvents.
Chemical properties: Potassium permanganate is a very strong oxidizing agent, thus it is used in many chemical reaction to obtain inorganic or organic chemical substances. The reason is due permanganate anion is formed by manganese in the oxidation estate +7, it is most oxidized state (or the higher positive charge) for the manganese atom. Thus, it can easily react until the other lesser oxidized number in reaction known as Redox (reduction - oxidation) where a chemical ion loss electrons and the other gain them. A very known Redox is the reaction of potassium permanganate to manganese dioxide:
KMnO4 + substance which oxidize → MnO2 + substance oxidized
This reaction can easily be observed because the dark purple solution of permanganate turns to a colorless to brown solution with a brow precipitate of manganese dioxide. The reaction can be prepared in acid or base.Uses: Potassium permanganate is used extensively in chemical industry, laboratories and other industries as an oxidizing. It is used in medicine as an antibacterial deodorant and anriseptic; a solution 0.1% can kill the majority of bacteria. Potassium permanganate is widely used as bactericide in the treatment of cattle and sheep diarrhea. Moreover, potassium permanganate is used in water treatment industry.
Health effects / safety hazards: Potassium permanganate is irritant to eyes and skin when concentrate. It is not flammable, however is a strong oxidizing which can react with many reducing agents or organic material.
|
Related Links: |
