Potassium Oxide Formula
Potassium oxide, also known as potash, potassium oxidopotassium or dipotassium oxide, is an inorganic compound used as an additive in the manufacturing of glasses, ceramic and other materials.
Formula and structure: Potassium oxide molecular formula is K2O. The molar mass is 94.196 g/mol. This molecule is formed by two potassium cations K+2 and one oxygen anion O-2. The two cations are bound to the anion through ionic bonds. The molecule has an antifluorite crystalline structure, with one cation bound to 4 anions and each anion bound to 8 cations. Its chemical structure can be written as below, in the common representations used for organic molecules.
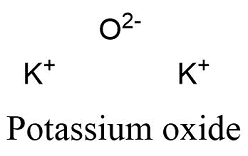
Occurrence: Potassium oxide is not found in nature. It is prepared from one of the methodologies described below.
Preparation: Potassium oxide is produced by different reactions. The easiest method is with elemental potassium in which a stream of oxygen gas is passed:
2K + O2 → K2O
Another method is the reaction of potassium peroxide with elemental potassium or just heating the potassium peroxide to promote the decomposition:
2K + 1/2 K2O2 → K2O
K2O2 → K2O + 1/2 O2
Physical properties: Potassium oxide is a yellow, odorless solid. The density of this oxide is 2.32 g/L. Its melting point is 740 °C and above this temperature, it decomposes. It is soluble in some organic solvents.
Chemical properties: Potassium oxide is known to react in water to form KOH through an exothermic reaction that can in some cases be violent:
K2O + H2O → 2 KOH
This reaction can also be promoted by the humidity of the environment, so the compound should be storage adequately.
Uses: Potassium oxide is used as fertilizer and as a component of other mixtures destined to the agriculture care. It is also used in the manufacturing of cement and some glasses and ceramic.
Health effects / safety hazards: Potassium oxide causes irritation in eyes and skin. It is corrosive and can react violently with water.
|
Related Links: |
