Potassium cyanide Formula - Uses, Properties, Structure and Formula
Potassium cyanide is a very poisonous inorganic cyanide salt, sometimes used in place of the more common sodium cyanide.
Formula and structure: The chemical formula of potassium cyanide is KCN. Its molecular formula is CKN and its molar mass is 65.12 g/mol. It is a salt which is composed of the potassium (K+) cation and the cyanide (CN-) anion, in which the carbon atom is triply bonded to the nitrogen atom and has a negative charge. Solid KCN exists in a similar cubic crystalline structure as sodium chloride (NaCl).
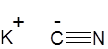
Preparation: Potassium cyanide is prepared industrially by a couple of methods involving potassium hydroxide (KOH). The reaction of hydrogen cyanide with an aqueous solution of KOH, followed by removal of water gives solid KCN.
HCN + KOH → KCN + H2O
KCN can also be prepared by reacting formamide (HCONH2) with KOH:
HCONH2 + KOH → KCN + 2H2O
Physical properties: Potassium cyanide is a white or colorless crystalline solid, which has a faint odor of almonds (due to the cyanide ion). It has a density of 1.52 g/mL and a melting point of 634.5 °C. It is a highly water soluble and deliquescent solid (which absorbs water from air and dissolves).
Chemical properties: The chemical properties of KCN are very similar to those of sodium cyanide (NaCN). It decomposes slowly in air (by absorbing the atmospheric moisture or carbon dioxide) and decomposes rapidly on heating to release toxic hydrogen cyanide gas (HCN). It also reacts with acids to form HCN. Thus, KCN crystals must be stored in very dry and acid-free conditions.
Potassium cyanide can be rendered safe by reacting it with a base or hydrogen peroxide (H2O2) to form the less harmful cyanate derivative (KOCN).
KCN + H2O2 → KOCN + H2O
Uses: KCN is used in the mining industry for extraction of gold and other metals. Its main uses are in organic synthesis, and in the preparation of many useful chemicals, plastics and pharmaceuticals. It is also used in electroplating, photographic developing, fumigation, and as an insecticide.
Health effects/safety hazards: Potassium cyanide is highly toxic and a potent poison, in a similar manner as sodium cyanide. If swallowed, it is a fast acting poison even in small doses. Exposure to the solid KCN can also be dangerous as it slowly releases the toxic HCN gas in air, which is toxic by inhalation.
|
Related Links: |
Related Topics
Hydrocyanic Acid Formula - Hydrocyanic Acid Uses, Properties ...
Chemistry Formulas
Formulas: Physics Formulas and Math Formulas
General Chemistry topics
