Potassium Bromide Formula
Potassium bromide is an inorganic salt used in past as anticonvulsant. Today it is still used to produce veterinary medicines.
Formula and structure: Potassium bromide chemical formula is KBr and the molar mass is 119.00 g mol-1. The structure of the salt is formed by one cation K+ and one anion Br-. The crystalline structure is an octahedral formed by one bromine anion surrounded by six potassium cation and vice versa. Its chemical structure can be written as below, in the common representations used for organic molecules.
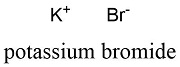
Occurrence: Potassium bromide is not found in nature.
Preparation: Potassium bromide is prepared by the reaction of potassium carbonate with iron (III) bromide:
4 K2CO3 + Fe3Br8 → 8 KBr + Fe3O4 + 4 CO2
Physical properties: Potassium bromide is an odorless, white crystalline solid, with a sweet taste and with a density of 2.74 g mL-1. The melting point of the salt is 734 °C, while the boiling point is 1435 °C. Potassium bromide is highly soluble in water and slightly soluble in diethyl ether. It is hygroscopic.
Chemical properties: Potassium bromide is formed by two ions with high difference of electronegativity: potassium cation K+ and bromine anion Br-. Due to great difference the ionic bond is stronger but at the same time, the molecule can easily dissociate in water to form the ions. The formation of bromine anion can be used to produce some salts as silver bromide, which precipitates in water and can be recovered through filtration:
KBr(aq) + AgNO3(aq) → AgBr(s) + KNO3(aq)
Uses: Potassium bromide was used as medicine to combat the convulsion in century XIX and first part of XX. It was also used as a sedative. Today, it only remains as medicine to treat epilepsy of veterinary use. It is commonly used in the technique of Infrared spectroscopy due to the crystal formed is transparent and does not have optical absorption. Potassium bromide is added in some formulation to reveal photographies.
Health effects / safety hazards: Potassium bromide is an irritant to eyes. In large quantities. Its ingestion can cause somnolence, delirium and psychosis. It is not flammable.
|
Related Links: |
