Nitrous Acid Formula - Nitrous acid Uses, Properties, Structure and Formula
Nitrous acid (HNO2) is a weak acid that exists in solution or as nitrite salts only.
Formula and structure: The chemical formula of nitrous acid is HNO2 and its molar mass is 47.013 g/mol. The structure of HNO2 is shown below. It is a planar molecule with the nitrogen atom attached to two electronegative oxygen atoms through a single bond and a double bond (in resonance, depending on the solution). One of the oxygen atoms is attached to the hydrogen atom, and holds it quite strongly, thus making HNO2 a weak acid.
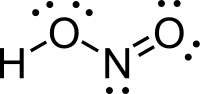
Also, due to the presence of a double bond, nitrous acid can exist as cis and trans isomers in the gas phase, with the stable trans-isomer being predominant at room temperature.
Occurrence: Nitrous acid is produced naturally in the earth's atmosphere by the reaction of nitric oxide (NO) and water, and helps in regulating the ozone content in the atmosphere.
Preparation: HNO2 can be produced by dissolving dinitrogen trioxide (N2O3) in water as shown below:
N2O3 + H2O → 2 HNO2
Another common method of preparing nitrous acid is by reacting sodium nitrite (NaNO2) with mineral acids (such as HCl, HBr, etc.).
NaNO2 + H+ ----> HNO2 + Na+
Physical properties: Nitrous acid is typically a pale blue solution, with a density of about 1 g/mL. It is only present as a solution in water or as nitrite salts.
Chemical properties: Nitrous acid is a monobasic acid, so it releases only one proton (H+) in solution. Moreover, it is a weak acid, so it does not dissociate fully in water and remains in equilibrium with the dissociated molecules. Also, like other acids, nitrous acid reacts with bases to form salts.
Nitrous acid is an unstable molecule and decomposes readily in solution by either one of these pathways.
2HNO2 → NO2 + NO + H2O
4HNO2 → 2HNO3 + N2O + H2O
Uses: Nitrous acid has several uses in industry. It is widely used in the preparation of diazonium salts, which then react with aromatic amines and phenols to form azo-dyes.
HNO2 + ArNH2 + H+ → ArN = NAr + 2 H2O (Ar is an aryl group)
Safety hazards/ health effects: Nitrous acid is a powerful oxidizer, and explodes when it comes in contact with phosphorus trichloride (PCl3). It is not severely toxic, however, it affects respiratory health and causes some irritation symptoms in asthmatics.
|
Related Links: |
