Nitrogen gas Formula
Nitrogen gas is a diatomic, colourless gas that forms the 78% of the atmosphere of the Earth.
Formula and structure: The nitrogen gas chemical formula is N2. The molar mass is g/mol. The molecule is formed by two nitrogen atoms bound through a triple bond. All the triple bonds are formed by one sigma bond and two pi bonds, forming a linear molecule. Its chemical structure can be written as below, in the common representations used for organic molecules.
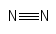
Occurrence: Nitrogen gas is present in the nature, it is found in the atmosphere and in all living organisms. Since atomic nitrogen does not exist in nature, every reference to the nitrogen presents in nature is referred to the diatomic nitrogen gas.
Preparation: Nitrogen can be prepared for commercial uses to the cryogenic distillation of the air. This distillation results in the formation of nitrogen gas and also oxygen and argon gas as side-products. The nitrogen resulting from this distillation is liquid, although can be pressurized to form the nitrogen gas. It can also be obtained from the combustion of natural gas or propane.
Physical properties: Nitrogen gas is a colorless and odorless gas or liquid, depending on the temperature. At room temperature, it is a gas. Its melting point is -210°C, while its boiling point is -196 °C. Nitrogen gas is slightly soluble in water and alcohol.
Chemical properties: Nitrogen gas is a super stable molecule. Many nitrogen compounds tend to decompose to form this gas due to its great stability. The reason behind this stability is the triple bond formed between the two nitrogen atoms, through this electronic arrangement, each nitrogen atom reach the eight electron valence rule (rule of octet). The bond distance in nitrogen molecule is extremely short, showing the triple bond is strong, requiring a high energy (225 kcal/mole) to break it.
Uses: Nitrogen gas is largely used to perform reactions in chemical industry and laboratory under inert atmospheres. The low reactivity of this gas makes possible to perform chemical reaction without the interference of the oxygen of the air. Nitrogen is also used in the formulation of fresheners and aerosols and pesticides. Nitrogen gas is also a reactant in the manufacture of ammonia, nitrate and other nitrogen compounds.
Health effects / safety hazards: Nitrogen gas is non-toxic and it is safe for inhalation. It is non-flammable and non-reactive. Although, if the containers of nitrogen gas are heated, they can explode.
|
Related Links: |
