Nitric Acid Formula - Nitric Acid Uses, Properties, Structure and Formula
Nitric acid is a very strong and corrosive mineral acid, also called aqua fortis or spirit of niter.
Formula and structure: The chemical formula of nitric acid is HNO3. Its molecular formula is written as NHO3 and its molar mass is 63.01 g/mol. The chemical structure of nitric acid is shown below, with its resonance forms:
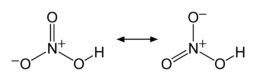
The HNO3 molecule is planar with the nitrogen attached to three oxygen atoms, one of which holds the proton. Two of the N-O bonds are equivalent and show resonance with double bond character.
Preparation: Nitric acid is prepared by reaction of nitrogen dioxide (NO2) with water.
3 NO2 + H2O → 2 HNO3 + NO
The nitric oxide (NO) by-product usually gets oxidized again by the oxygen in air to produce additional nitrogen dioxide starting material.
The commercial production of nitric acid is by oxidizing anhydrous ammonia to nitric oxide, in the presence of platinum catalyst at a high temperature (Ostwald process).
Physical properties: Nitric acid is a liquid with an acrid, pungent and suffocating odor. There are different concentrations of nitric acid available, and they are colorless, yellow or red accordingly. The industrial grade is about 68% in water, commercial grade is between 52% and 68%, fuming nitric acid is 86% or higher, while concentrations above 95% are called white fuming or red fuming nitric acid.
Chemical properties: Nitric acid is a strong, monoprotic acid. It readily forms solid hydrates such as the monohydrate (HNO3·H2O) and the trihydrate (HNO3·3H2O). Nitric acid can be decomposed by heat or light as shown below:
4 HNO3 → 2 H2O + 4 NO2 + O2.
It is a powerful oxidizing agent, and reacts violently with many non-metallic compounds. It also reacts with metals to dissolve them, form metal oxides, etc.
Uses: Nitric acid is widely used for the production of fertilizers, such as ammonium nitrate, and polymers (eg. Nylon). It is an excellent nitrating agent (introduces a nitro group), in combination with sulfuric acid. It is also used as oxidizer in liquid-fueled rockets.
Health hazards/ health effects: Nitric acid is a corrosive acid which can cause severe skin burns. Being a strong acid and oxidizer, it can completely decompose tissues. Even dilute forms can cause burns and stain the skin yellow by reacting with the skin's proteins. The pungent fumes are also very irritating and damaging to eyes, throat and mucous membranes.
|
Related Links: |
