Nitrate Formula
Nitrate is an inorganic ion that is the anion forming many nitrate salts. These salts are present in a wide number of compounds in nature and are relevant for the chemical industry.
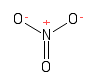
Formula and structure: The nitrate chemical formula is NO3-. The molar mass is 62.04 g/mol. Nitrate is the conjugate base of nitric acid HNO3. The three oxygen anions are bound to a centre nitrogen cation through one double and two single bonds. In reality, because the effect of resonance the three bonds are equivalent, with an average behaviour between single and double bond. The geometry of the molecule is trigonal planar. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Nitrate is found in nature as the anion forming many salts. Some of these salts are lead (II) nitrate, silver (I) nitrate, sodium nitrate and calcium nitrate. According to the salt, it can be found in large amounts of ores and minerals in the planet, as the case of nitratine. Nitrate is important and can be found in soils.
Preparation: Nitrate is preparing for methods that vary according to the target salt. In general, they are prepared from a reaction between nitric acid and the corresponding metal or salt. One of these examples is the preparation of lead (II) nitrate:
Pb (metal) + 4 HNO3 → Pb(NO3)2 + 2 NO2 + 2 H2O
Physical properties: The physical properties of nitrate vary with the combination of cation to form the nitrate salt. Most of them are crystalline solids and all the nitrate are soluble in water.
Chemical properties: The nitrate anion is well-known and used as an example of the resonance effect, in which the presence of double and single bonds between the same atoms creates a new type of bond shared by all the atoms. In the case of nitrate, the three N-O bonds have the same distance and share 1/3 of the -1 charge over the total NO3-.
Uses: Nitrate salts are largely used in food industry. They are also present is many vegetables as the spinach and beetroot. Nitrates are also used as fertilizers for being a source of nitrogen. Particularly, sodium nitrate and ammonium nitrate are the most used as fertilizers. Nitrate salts are also used as component of explosives for the military industry.
Health effects / safety hazards: Nitrate salt can be toxic and although many of them are found in food and vegetables, other can be extremely toxic for human. Nitrate is a anion that form part of many explosive, so the reactions involving this anion should be handle carefully. Nitrate is also a strong oxidizing agent and can react violently in contact with other chemical compounds.
|
Related Links: |
