Molar Mass Formula
Molar Mass Formula
Molar mass is the mass of one mole of a substance. The mass will be represented in grams while a mole of a substance is defined as 6.02 x 1023 representative particles (Avogadro's number) of that substance.
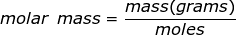
Molar Mass Formula Questions:
1. Calculate the molar mass of a substance if 0.235 moles has a mass of 45.67 grams.
Answer:
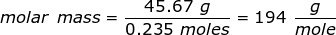
2. Calculate the number of grams of a substance if 1.35 moles has a molar mass of 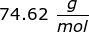 .
.
Answer:
The equation of molar mass will need to be rearranged to solve for the mass.
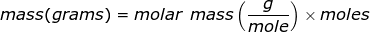
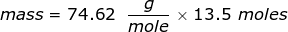
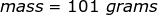
|
Related Links: Molar Mass Quiz |
