Methane Formula
Methane, also known as methyl hydride or marsh gas, is a gas extensively found in nature, which is very used as combustible.
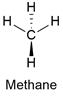
Formula and structure: The methane chemical formula is CH4 and is also written as Met. Its molar mass is 16.043 g mol-1. The molecule is the simplest organic compound and it is considered the base of more complex organic molecules. The methane molecule has a tetrahedral geometry with 4 hydrogen bound to a carbon atom. The carbon atom has a sp3 hybridization, thus it can be explained its geometry. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Methane is widely found in nature. It is present in soils, seas and air. Methane is the main components of natural gas and it can also be recovered from petroleum wells in soils and seas. This gas is produced by organic sediments in decomposition by the action of micro-organism, which originated reserves of methane in soils. Methane present in atmosphere is part of the greenhouses gases that contribute with the global warming.
Preparation: Methane is mostly extracted from natural gas due it represents 87% of the total composition material. However, there are some processes to produce methane through organic or inorganic synthesis; for example through the reaction between carbon monoxide and hydrogen. It is the simplest route to produce methane and it is used a nickel catalyst and high temperature:
CO + 3H2 → CH4 + H2O
Other route is the reaction of water and carbon dioxide in a reaction that uses a ruthenium electrode. In nature, methane from decomposition of organic matter by bacteria and fughi is called methanogenesis and it is actually very used to produce methane from biomass in biotechnological processes:
CO2 + 4H2 → CH4 + 2H2O
Physical properties: Methane is a colorless and odorless gas. Its melting and boiling point are -183 ºC and -161 ºC, respectively. Its density is 0.716 g mL-1. Methane can easily ignite forming vapors lighter than air. It is not soluble in water, but is soluble in ethanol, ether, benezene, toluene and methanol.
Chemical properties: Methane is the most simple organic compound, so it is only formed by one carbon atoms and 4 hydrogen resulting in very light molecule that does not have the enough intermolecular interaction to form a liquid or solid. The total dipole moment in methane molecule is 0 impeding a high reactive of the specie and resulting in a very weak acid in solution.
Uses: Ethane is used as combustible in homes, mainly in water heaters and ovens. It is also an industrial combustible to turbines for generating electricity and also as a fuel alternative in automobiles.
Health effects / safety hazards: Methane is heavier than air, so that in high concentration it may cause asphyxiation by displaced the oxygen. It is extremely flammable and can ignite even at low temperatures. It may also explode by contact with strong oxidizing agents and halogens such as chlorine dioxide and nitrogen trifluoride or in contact with boiling water.
|
Related Links: |
