Magnesium sulfate Formula - Magnesium sulfate Uses, Properties, Structure and Formula
Magnesium sulfate is a widely used inorganic compound which is also called as Epsom salt or bath salts.
Formula and structure: The chemical formula of magnesium sulfate is MgSO4 and its molar mass is 120.366 g/mol. It also commonly exists as the monohydrate form (with one molecule of water) which has the chemical formula of MgSO4.H2O, and molar mass of 138.38 g/mol. Magnesium sulfate is a salt composed of the bivalent magnesium cation (Mg2+) and the sulfate anion (SO42-), in which the central sulfur atom is attached to four oxygen atoms through two single and two double bonds. Solid MgSO4 adopts an orthorhombic crystalline structure.
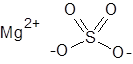
Occurrence: Magnesium sulfate occurs in nature in its hydrated forms in different minerals. It is most commonly found in its heptahydrate form, as the mineral epsomite (MgSO4·7H2O), or Epsom salt. It also occurs in its monohydrate form as the mineral kieserite (MgSO4·H2O).
Preparation: The naturally occurring MgSO4 minerals are the major source of magnesium sulfate after mining, processing and dehydration of the hydrated forms. It can also be prepared chemically by the reaction of sulfuric acid with either magnesium carbonate, magnesium hydroxide or magnesium oxide.
Physical properties: Magnesium sulfate exists as an odorless, white crystalline solid with a density of 2.66 g/mL, and melting point of 1,124°C. The monohydrate form of magnesium sulfate has a density of 2.45 g/mL, and melting point of 200°C.
Chemical properties: MgSO4 is highly soluble in water, and is also extremely hygroscopic (absorbs moisture from atmosphere). It can absorb large amounts of water in its anhydrous form (dry, without any water molecules), and is an important laboratory desiccant. It is stable at normal conditions. When heated to high temperatures, it decomposes to give toxic fumes of sulfur oxides.
Uses: Magnesium sulfate has many medical and household uses such as in bathing salts, beauty products, anticonvulsants, muscle relaxants, laxative and electrolyte source (of Mg, an essential nutrient), among others. It also has important industrial applications such as electrolyte, bleaching agent, desiccant, absorbent, fertilizer, brewing salt, etc.
Health effects/safety hazards: Magnesium sulfate is not considered toxic at recommended dosages and is an important medicine. However, when ingested in high concentrations, it can cause a condition called hypermagnesemia as well as symptoms such as abdominal pain, diarrhea and vomiting. When heated to decomposition, it releases fumes of sulfur oxides which are toxic and corrosive.
|
Related Links: |
