Magnesium sulfate salt (Epsom salt) Formula
Magnesium sulfate, also known as Epsom salt, is an inorganic salt mainly used in agriculture as fertilizer. It is also used by the pharmaceutical industry.
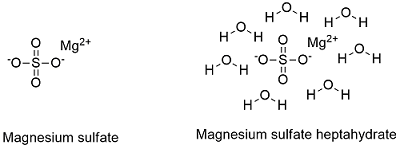
Formula and structure: Magnesium sulfate has three known form: anhydrous, monohydrated and heptahydrated form and its chemical formula are: MgSO4, MgSO4.H2O and MgSO4.7H2O and the molar mass are 120.361 g mol-1, 138.361 g mol-1 and 246.361 g mol-1, respectively. The most common form is the heptahydrated salt which is called Epsom salt which has an orthorhombic structure. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Magnesium sulfate is a common mineral in nature. It is mostly found in caverns and other geological formations. The most common form, the Epsom salt, comes from the mineral Epsomite and is found together with the minerals alunogen Al2(SO4)3.17H2O and gypsum CaSO4.2H2O.
Preparation: Most of magnesium salt is obtained from the mineral ores. The Epsom salt comes from the Epsomite, while the magnesium sulfate monohydrate is recovery from the mineral Kieserite. Although the monohydrated and anhydrous salts are also obtained from the dehydration of Epsom salt by heating.
Physical properties: Magnesium sulfate is an odorless, colorless to white crystalline solid (or amorphous solid when it is anhydrous). It has a bitter taste and its density is 1.68 g mL-1 and 2.66 g mL-1 in the heptahydrated and anhydrous form respectively. It heptahydrated salt decomposes above 150 ºC while the anhydrous forms has a decomposition temperature of 1136 ºC. It is highly soluble in water and soluble in ethanol and glycerin. Magnesium sulfate is insoluble in acetone and ether. It is hygroscopic.
Chemical properties: Magnesium sulfate is essential for biochemical systems in prokaryotes and eukaryotes due magnesium actives many enzymes to promote the vital reactions in organism, for example: nucleic acid synthesis or carbohydrate metabolism. Moreover, magnesium sulfate participates in REDOX reactions through the reduction of sulfur helping in the process of fertilization in plants.
Uses: Magnesium sulfate is very used in pharmaceutical industry and also as a drug, It is used in medicine as an electrolyte replenisher, anticonvulsant and mainly in the pre-eclampsia treatment to reduce the frequency of contractions in pregnant women. It can be used in the treatment against the magnesium deficiency, although the magnesium chloride is more used. Magnesium sulfate is used as fertilizer to promote the grow of roots and it is also a raw material or additive in chemical industry to produce fabrics, leather or also as desiccant.
Health effects / safety hazards: Magnesium salt also present risk when consumed in larger quantities. It is not flammable or reactive with other chemical compounds.
|
Related Links: |
