Magnesium Phosphate Formula
Magnesium phosphate is the name of a group of salts that contain the ions magnesium and phosphate in different proportion. These salts are used in the food industry as an acidity regulator.
Formula and structure: The chemical structure of the magnesium phosphate salt is basically formed by 1-3 magnesium cations Mg2+ and 1-2 phosphate ions HPO4-, PO43-. The salts are frequently hydrate, so the structure is surrounded by several molecules of water. The name of the main magnesium phosphate salts are: monomagnesium phosphate (Mg(H2PO4)2 that has a molar mass of 120.28 g/mol; dimagnesium phosphate MgHPO4, that has a molar mass of 218.28 g/mol and trimagnesium phosphate Mg3(PO4)2), that has a molar mass of 262.85 g/mol. Their chemical structures can be written as below, in the common representations used for organic molecules.
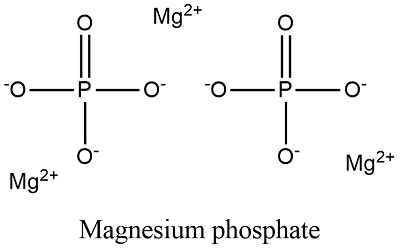
Occurrence: These phosphate salts are found in minerals. For example, dimagnesium phosphate is commonly found in its trihydrate form as being part of newberyite, besite or natrophilite.
Preparation: Magnesium phosphate salts can be prepared by precipitation used a solution of tribasic sodium phosphate and magnesium chloride:
Na3PO4 + 3 MgCl2 → 3 Na+ + 6Cl- + Mg3PO4
However, the most common reaction for producing monomagnesium phosphate is magnesium oxide with phosphoric acid.
MgO + H3PO4 → MgH2PO4 + H2O
with the monomagnesium phosphate salt can be produced the dimagnesium phosphate salt and phosphoric acid is formed as a side product:
Mg(H2PO4)2 + 3 H2O → Mg(HPO4).3H2O + H3PO4
Physical properties: Monomagnesium, dimagnesium and trimagensium phosphate salts are white, odorless, crystalline powder. The trimagnesium salt has a melting point of 1184 ºC, it is insoluble in water but it is soluble in saturated solution of NaCl.
Chemical properties: Most of phosphates salts are soluble in water, however, magnesium salts are not soluble due to the cation magnesium uses to form insoluble compounds, it is also the case of magnesium hydroxide or magnesium sulfate. The phosphate salts are good as acidity regulator due to the phosphate ion resists the change of pH in a solution.
Uses: Magnesium phosphate salts are widely use in the food industry as a acidity regulator. They are also used in the agricultural field, as a fertilizer for crops and soil conditioner.
Health effects/safety hazards: Magnesium phosphate salts are listed as safe substances in the US FDA.
|
Related Links: |
