Magnesium Nitrate Formula
Magnesium nitrate, also called nitromagnesite, is a inorganic compound used as fertilizer due to it is a cheap and high source of nitrogen. It is also used as a dryer agent.
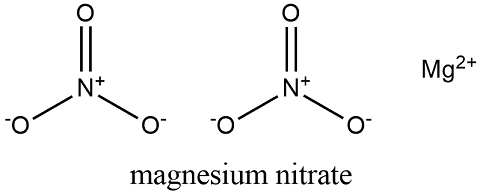
Formula and structure: The magnesium nitrate chemical formula Mg(NO3)2 and the molar ma.ss is 148.32 g mol-1. This salt is commonly found in its hydrated forms: dihydrate (Mg(NO3)2.H2O) and hexahydrate (Mg(NO3)2.6H2O) with the molar mass of 184.35 g mol-1 and 256.41 g mol-1 respectively. This salt is formed by one magnesium cation (Mg2+) and two nitrate anions (NO3-). Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Magnesium nitrate is found in nature in its hexahydrate form. It can be extracted from mines and caverns where it is along with other magnesium minerals.
Preparation: Magnesium nitrate can be prepared by the reaction of magnesium oxide with nitric acid:
MgO + 2HNO3 → Mg(NO3)2 + H2O
This reaction can also be prepared using magnesium carbonate or hydroxide in nitric acid.
Physical properties: Magnesium nitrate is white crystalline solid, highly hygroscopic. Its density is 2.3 g mL-1 (anhydrous), 2.026 g mL-1 (dihydrate) and 1.464 g mL-1 (hexahydrate). The melting point is 129 ºC (dihydrate) and 88.9 ºC (hexahydrate) and the boiling point is 330ºC with decomposition. It is highly soluble in water and slightly soluble in ammonia.
Chemical properties: Magnesium nitrate is highly soluble in water, thus it can be used as a desiccant agent to bind water molecules in non-aqueous solution. Anhydrous magnesium nitrate cannot be obtained from heating the hexahydrate salt, it is rather produced from the synthetic routes described above. It can be used to prepared magnesium hydroxide according to the reaction:
Mg(NO3)2 + 2 NaOH → Mg(OH)2 + 2 NaNO3.
Uses: Magnesium nitrate is using as dryer agent in some specific industrial processes such as the preparation of nitric acid. It is used as a fertilizer because its high content of nitrogen and magnesium.
Health effects / safety hazards: Magnesium nitrate is a oxidant, thus it may intensify fire. It can cause serious skin and eyes irritation and also respiratory tract damage.
|
Related Links: |
