Magnesium Chloride Formula
Magnesium chloride, also known as magnesium dichloride, is an inorganic salt that is found in many organisms to accomplish essential functions.
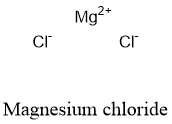
Formula and structure: The magnesium chloride chemical formula is MgCl2. The molar mass is 95.21 g/mol. This salt is found in as a dihydrated salt with the formula MgCl2.H2O and molecular mass 203.31 g/mol, being it the most common form. The molecule is formed by one cation ferrous Mg2+ and two chloride anions Cl-. The structure is formed by a octahedral geometry, with 8 chloride anions surrounded a magnesium cation. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Magnesium chloride is found in nature. It can be extracted from sea water and salty lakes. One of the main sources is the Dead Sea in the Middle East. It is also found as solvated salt in organisms of all levels (bacteria, animals and human).
Preparation: Magnesium chloride is formed by the reaction of hydrochloric acid and magnesium oxide. It can be obtained from adding the hydrochloric to the seawater, precipitating the magnesium chloride. It can also be prepared from magnesium ammonium chloride reacting with hydrochloric acid.
Physical properties: Magnesium chloride is a white, odourless, crystalline solid. The density varies according to the hydration of the salt, being 2.32 g/mL for the anhydrous and 1.59 g/mL for the hexahydrate salt. The melting point is 714 °C for the anhydrous and 1412 °C is the boiling point. Magnesium chloride is soluble in water and acetone.
Chemical properties: Magnesium chloride is an essential salt to the body of organisms because it can be solvated in two important ions: Mg2+ and Cl-. The magnesium cation Mg2+ has an important biochemical role as a cofactor of many enzymes and to guarantee the ionic biological balance. Magnesium chloride is a Lewis acid meaning that even when it is an salt, it can also be used as an acid due to it is able to accept electrons.
Uses: Magnesium chloride is used as a component in the production of adhesives, cleaning agents, agricultural fertilizers, animal food and human food. It is also used as the main reactant in the production of magnesium metal. Magnesium chloride is also used as a catalyst in some chemical reactions. Magnesium chloride can be used as a component of fertilizers.
Health effects / safety hazards: Magnesium chloride can cause eyes irritation but I general is not toxic. It is not flammable.
|
Related Links: |
