Iron (III) Carbonate Formula
Iron (III) carbonate, also known as ferric carbonate, is an inorganic salt that is found in some minerals. It is a unstable compound.
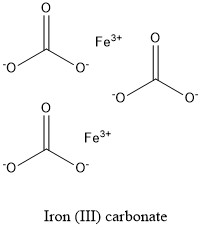
Formula and structure: Iron (III) carbonate chemical formula is Fe2(CO3)3. The molar mass is 291.72 g mol-1. The molecule is formed by two cations Fe3+ and three carbonate anions CO32-. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Some traces of iron (III) carbonate can be found in some minerals along with the iron (II) carbonate. But in general, it is too unstable to be isolated.
Preparation: There are not routes to prepared iron (III) carbonate described in literature.
Physical properties: Physical properties of iron (III) has not been evaluated due to its instability and quickly decomposition. It is hygroscopic and decomposes in water.
Chemical properties: The iron (III) carbonate suffers a reaction of decomposition that takes place quickly and does not allow to isolate the compound. The decomposition takes place through the reaction:
Fe2(CO3)3 → Fe2O3 + 3CO2
This reaction is thermodynamically favored, so the formation of the product is fast and reactant cannot be found.
Uses: Iron (III) carbonate is not found an isolated compound, so its uses have not been reported.
Health effects / safety hazards: Iron (III) carbonate is not found an isolated compound, so that its health effects and safety hazards have not been studied.
|
Related Links: |
