Imidazole Formula
Imidazole is a versatile heterocycle largely used in synthesis compounds with a wide variety of biological activities such as antifungal, antiparisitic, antibacterial and antiviral medication.
Formula and structure: The molecular formula of imidazole is C3H4N2 and its molar mass is 68.08 g mol-1. It is a five-members aromatic heterocycle where two nitrogen atoms substituting two CH- groups. The two nitrogen atoms are non-adjacent positions. Imidazole is a planar molecule; so there is an unshared electron pair under a nitrogen in a sp2 orbital. This electron pair is responsible by various of chemical properties of imidazole, such as the basicity of molecule. Its chemical structure, including the resonance structures, can be written as bellow:
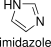
Occurrence: Free imidazole is not frequently found in nature. However, the imizadole derivatives are extended in nature, for examples: the amino acid histidine or the compounds histamine, bezimidazole and some alkaloids have the imidazole ring in their structures.
Preparation: Imidazole can be prepared by various methods. The general synthesis of imidazole uses oxaldehyde (also known as glyoxal) and formaldehyde in ammonia; however, it can be modified the reactants in other to obtain different substituted imidazoles or imidazole derivatives.
OCHCHO + H2C=O + NH3 → imidazole
Physical properties: Imidazole is a white or colorless solid. It can show a prismatic crystal appearance. Imidazole is highly soluble in water, ethanol, ethyl ether and chloroform and it is insoluble in non-polar solvents. In water, it produces an alkaline solution. Its melting point is 88-91 ºC and its boiling point is 256 ºC.
Chemical properties: Imidazole is a 5-member rings that show two tautomeric structures due the positive charge can be located in both of the nitrogen atoms, thus the hydrogen binds to the nitrogen atom can be removed easily because the lesser electron density of this nitrogen atom. On the other hand, the other nitrogen atom has an unshared electron pair in a sp2 orbital that can accept protons. Consequently, imidazole shows an amphoteric behavior: it can act as a weak base or a weak acid.
Uses: Imidazole is used as raw material by the pharmaceutical industries for manufacturing anti-fungal drugs such as ketoconazole and clotrimazole or the bactericide imazilil and prochloraz. Similarly, the pesticide industries use the imidazole as intermediary in the synthesis of some pesticides and insecticides. It can also be used as corrosion inhibitor of metals such as copper. Moreover, imidazole has found application as epoxy curing agent, to improve the electrical properties in electronic devices.
Owing to the fact an imidazole ring is present in the aminoacid histidine, imidazole solutions is extensively apply in molecular biology techniques to purify recombinant proteins through metal affinity chromatography (IMAC). The proteins can be expressed with a histidine tails to promote the retention in a chromatography column that contains nickel ions and then, imidazole solution are used to displace the histidine tagged protein.
Health effects/safety hazards: Imidazole is harmful by oral ingestion. It is also corrosive and can cause severe skin burns and eye damage.
|
Related Links: |
