Hydrogen Sulfide Formula
Hydrogen sulfide, also known as sewer gas, hydrosulfuric acid or sulfhydric acid, is a toxic gas used as a precursor in the production of elemental sulfur.
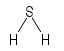
Formula and structure: The hydrogen sulfide chemical formula is H2S. The molar mass is 34.08 g/mol. The molecule is formed by two protons H+ and one sulfur anion S2-. The two protons are bound to the sulfur centered atom through a single covalent bond. The structure is similar to the water molecule, forming a V between the three atoms with an angle of 92°. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Hydrogen sulfide is found in nature as produced by bacteria through the cleavage of organic matter. It can also be found in volcanic gases, crude oil and natural gas. In some deep water well as hot springs, hydrogen sulfide can be also found. Hydrogen sulfide is part of the biogeochemical cycle of sulfur on Earth. It is also biosynthesized by human in small amounts.
Preparation: Hydrogen sulfide can be extracted from natural gas or other gas containing it in its mixture. It can also be prepared with the reaction of hydrogen gas and sulfur solid at high temperatures:
1/2 S8 + 4 H2 → 4 H2S
The last method is not largely used and it is more common the inverse reaction of sulfide hydrogen with several catalysts to recover sulfur solid.
Physical properties: Hydrogen sulfide is a colourless gas with a pungent, like rotten eggs odour. The density of this salt is 1.36 g/mL. Its melting point is -82 °C and the boiling point is -60 °C. It is slightly soluble in water and it is not soluble in other in other organic solvents. Hydrogen sulfide is corrosive and it is heavier than air, so it can accumulate at the bottom of close spaces.
Chemical properties: Hydrogen sulfide is highly susceptible to suffer reduction or oxidation reactions; consequently it is classified as a highly reactive compound. It can reduce some bases and can also be an oxidant, for example, when in contact with metals, it can form sulfide salts. Another interesting activity is the conductivity potential that hydrogen sulfide has high pressures.
Uses: Hydrogen sulfide is mostly used in the production of other sulfur compounds such as thioesters, metal sulfide salts and to produce sulfur. One of these reactions to form metal salts (sulfides and hydrosulfides) are:
H2S + KOH → KSH + H2O
KSH + KOH → K2S + H2O
Hydrogen sulfide is also used as an analytical chemistry standard in the determination of presence of inorganic cations such as Cu2+, Hg 2+ and Pb2+.
Health effects / safety hazards: Hydrogen sulfide is extremely toxic and poisonous. It cannot be inhaled due to the damage to the nervous system that it can cause. It can form explosive mixtures with oxygen, forming sulfur dioxide. It can cause the rupture of close containers where it is stocked. Hydrogen sulfide is flammable.
|
Related Links: |
