Formic acid Formula
Formic acid is the simplest carboxylic acid, also called methanoic acid.
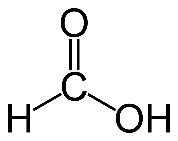
Formula and structure: The chemical formula of formic acid is HCOOH or HCO2H. Its molecular formula is CH2O2 and its molar mass is 46.02 g/mol. Its chemical structure is shown below. It consists of a single carboxylic acid group (COOH) attached to a hydrogen atom.
Occurrence: Formic acid occurs naturally in some insects, such as ants and bees.
Preparation: Formic acid is prepared through several routes. It is commonly prepared by reacting sodium formate with sulfuric acid. It is also prepared by the reaction of formamide (HCONH2) with sulfuric acid or by the hydrolysis of methyl formate (HCO2CH3), as shown below:
2 HCONH2 + 2H2O + H2SO4 → 2HCO2H + (NH4)2SO4
HCO2CH3 + H2O → HCO2H + CH3OH
Physical properties: Pure formic acid is a colorless liquid with a corrosive and pungent odor. Its density is 1.22 g/mL, melting point is 8.4 °C and boiling point is 101 °C. It is completely miscible with water
Chemical properties: Formic acid is a weak acid which behaves as a typical carboxylic acid and also has some aldehyde-like properties. It readily reacts with alcohols to form esters. Formic acid decomposes in the presence of acids or heat to give carbon monoxide (CO) and water. In the presence of platinum, it decomposes to give carbon dioxide and hydrogen instead.
Uses: Formic acid is mainly used as a preservative, antibacterial agent, artificial flavoring agent, and in household and industrial cleaning products. It is also used in leather tanning, dyeing, textile finishing, and rubber production.
Health effects/safety hazards: Dilute formic acid is not toxic and is used as a food additive. However, the concentrated acid is harmful and corrosive. Inhalation of the fumes can cause irritation to the mucous membranes, while skin/eye contact can cause blisters or burns. Swallowing large amounts or chronic exposure can lead to kidney damage.
|
Related Links: |
Related Topics
Acid Names Formulas
Chemistry Formulas
Formulas: Physics Formulas and Math Formulas
General Chemistry topics
