Enthalpy Formula
Enthalpy is a thermodynamic function that is equal to the total internal energy of the system plus the product of pressure and volume. The equation is as follows:
H = E + PV
where H is the enthalpy, E is the energy and PV is the pressure multiplied by the volume.
The total internal energy of a system is impossible to calculate, but changes in internal energy can. The changes involve heat transfer and work done (the expansion or contraction of a gas). Therefore the enthalpy of a reaction is noted as ΔH where the symbol Δ refers to the change. The ΔH of the reaction (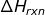 ) can be calculated in many different ways.
) can be calculated in many different ways.
1. If the work done by or on a system is zero (the volume of the container does not change) then the change in enthalpy is equal to the heat transfer (q), where  . In this equation m is the mass, s is the specific heat, and ΔT is the change in temperature.
. In this equation m is the mass, s is the specific heat, and ΔT is the change in temperature.
2. If the reaction is known, a table of 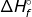 values can be used to calculate the
values can be used to calculate the 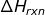 . The
. The 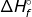 is called the heat of formation, and it refers to the heat is takes to form the substance from its elements.
is called the heat of formation, and it refers to the heat is takes to form the substance from its elements.

3. Hess's Law could be used to calculate the enthalpy of a reaction.
4. The 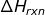 can be calculated by using the bond energies of the reactants and products.
can be calculated by using the bond energies of the reactants and products.
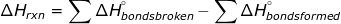
Enthalpy Formula Questions:
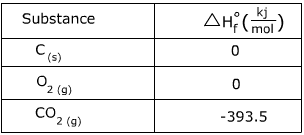
1. Calculate the heat of the following reaction using the table of 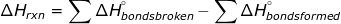 values.
values.
C(s) + O2 (g) → CO2 (g)
Answer:

The 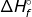 is called the heat of formation, and it refers to the heat is takes to form the substance from its elements. The
is called the heat of formation, and it refers to the heat is takes to form the substance from its elements. The 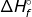 for C(s) and O2 (g) have values of 0 because they are in elemental form.
for C(s) and O2 (g) have values of 0 because they are in elemental form.
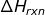 =
(-393.5)-(0 + 0) = -393.5kj
=
(-393.5)-(0 + 0) = -393.5kj
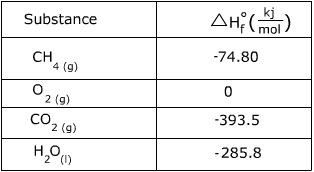
2. Calculate the heat of the following reaction using the table of 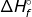 values.
values.
CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O(l)
Answer:

In this problem there are 2 moles of O2 and 2 moles of H2O so their 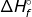 values must be multiplied by two.
values must be multiplied by two.
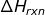 = [-393.5 + (2*285.8)] - [-74.80 + (2*0)] = -890.3kj
= [-393.5 + (2*285.8)] - [-74.80 + (2*0)] = -890.3kj
|
Related Links: |
