Dixygen Difluoride Formula
Dioxygen difluoride, also known as FOOF, fluorine peroxide or fluoroperoxyl fluoride, is an inorganic compound used as an oxidizer in rocket fuel systems.
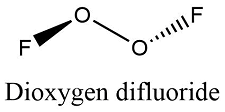
Formula and structure: The Dioxygen difluoride chemical formula is O2F2. The molar mass is 66.99 g/mol. The molecule is formed by two cations oxygen O+ and two fluorine anion F-. It has a structure similar to the hydrogen peroxide, with two oxygen bound through a O-O single bond and one fluorine anion joined to each oxygen atoms. The fluorine atoms remain in different special planes, with an angle of almost 180° between the fluorine and almost 109° between the F-O-O. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Dioxygen difluoride is not found in nature.
Preparation: Dioxygen difluoride can be prepared by the reaction of an aqueous solution of sodium hydroxide and fluorine gas:
NaOH + F2 → O2F2 + Na
It can also be obtained from the reaction of oxygen and fluorine gas at low temperatures:
O2 + F2 → O2F2
Physical properties: Dioxygen difluoride is an orange solid with a particular odor. The density is 1.45 g/mL. The melting point is -154 °C and the boiling point is -57 °C. It is insoluble in most of the solvents and reacts violently with them.
Chemical properties: Dioxygen difluoride suffers several reactions when exposed to the environment or to other chemical compounds. At temperature above -57 °C, it decomposes in oxygen and fluorine gas. It can react violently with most of the chemical components, so its use is very limited and it is extremely difficult to performed reaction with this component.
Uses: Dioxygen difluoride is used as an oxidizer in rocket fuel systems. It can also be used in small amounts in laboratories to research particular properties of components with similar structure or in the synthesis of analogs.
Health effects / safety hazards: Dioxygen difluoride is a poisonous gas. It is extremely toxic by inhalation. It can cause damage to the eyes and skin. It can react violently in contact with water. It can explode when heated.
|
Related Links: |
