Diphosphorus Pentoxide Formula
Diphosphorus pentoxide, also known as phosphorus (V) oxide or phosphoric anhydride is an oxide used as desiccant and dehydrating compound for organic synthesis.
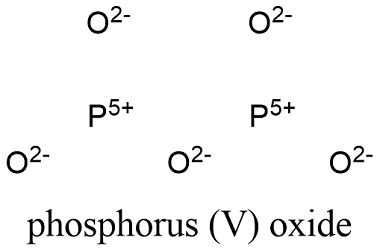
Formula and structure: Phosphorus pentoxide chemical formula is P2O5 and its molar mass is 141.94 g/mol, however it is also found as P4O10 with a molar mass of 283.886 g/mol. This oxide presents 4 known structures. The most studied is the P4O10 forming a hexagonal lattice or cage. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: phosphorous pentoxide does not exist as a free compound in nature.
Preparation: The main preparation method for phosphorous pentoxide is the reaction of tetraphosphorus with oxygen gas. It is burned producing the P4O10.
P4 + 5 O2 → P4O10
Physical properties: Phosphorus pentoxide is a white deliquescent solid. Its density is 2.39 g/mL. Its sublimation temperature is 360 ºC. It suffers an exothermic hydrolysis in water.
Chemical properties: Phosphorus pentoxide is a dryer and dehydrating agent due to exothermic hydrolysis that suffers in water, where energy of -177 kJ is released.
P4O10 + 6 H2O → 4 H3PO4
This reaction is also observed in the presence of amides, where the pentoxide can form a hydroxide and the amide is converted to nitrile.
P4O10 + RC(O)NH2 → P4O9(OH)2 + RCN
Uses: Phosphorus pentoxide is used as a dehydrating agent for halogenation with tetrabutylammonium halides. It is also used in the synthesis of some drugs and moieties sucha as poly(benzoxazole) and benzimidazole.
Health effects/safety hazards: Phosphorous pentoxide is very irritant and case cause serious damage in skin, eyes and respiratory tract. It is not flammable but can react vigorously with water or wet substances due to the exothermic hydrolysis that it suffers.
|
Related Links: |
