Copper (II) Chloride Formula
Copper (II) chloride, also known as cupric chloride, is a inorganic salt used as catalysts in chemical synthesis and is also used in the manufacturing of pyrotechniques for its blue/green colors.
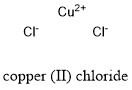
Formula and structure: Copper (II) chloride chemical formula is CuCl2 and the molar mass is 134.45 g mol-1. The dehydrated form has a molecular mass of 170.48 g mol-1. The structure of the anhydrous salt is formed by one cation Cu2+ and two anion Cl-. The structure is a distorted octahedron with a copper cation bound to six chloride anions. In this dehydrated form, it is formed by a Cu2+ centered atom, surrounded by 2 water molecules and two chlorine anions Cl-. Its chemical structure can be written as below, in the common representations used for organic molecules.
Occurrence: Copper (II) chloride, in its anhydrous or dehydrate forms, is found in some rare minerals as tolbachite and eriochalcite. This minerals are mostly found in fumaroles.
Preparation: Copper chloride is prepared from the reaction of metallic copper with chlorine:
Cu + Cl2 + 2 H2O → CuCl2
Copper (II) chloride can also be obtained by the reaction of copper (II) hydroxide or copper (II) carbonate with hydrochloric acid:
Cu(OH)2 + 2HCl → CuCl2 + 2 H2O
Physical properties: Copper (II) chloride is a blue-green solid when dehydrated and a yellow-brown solid when anhydrous. Copper (II) chloride density are 3.38 and 2.51 g mL-1for the anhydrous and dehydrate forms. The melting point of the anhydrous salt is 498 °C and above 993 ºC the anhydrous salt decomposes. Copper (II) chloride is soluble in water, ethanol, methanol and acetone.
Chemical properties: Copper (II) chloride can suffer a diverse range of reaction, which make it versatile to use in different application in chemical industry or chemical synthesis:
It can suffer a redox reaction in the presence of a reductant. The formed compounds include metallic copper or cuprous salt:
2 CuCl2 + SO2 + 2 H2O → 2 CuCl + 2 HCl + H 2SO4
With bases, copper (II) chloride can suffer a replacement to form copper (II) hydroxide:
CuCl2 + 2 NaOH → Cu(OH)2 + 2 NaCl
Uses: Copper (II) chloride can be used as a catalyst along with other chloride salts as the palladium (II) chloride. One of the uses is in the production of ethylene to acetaldehyde
C2H4 + PdCl2 + H2O → CH 3CHO + Pd + 2 HCl
Due to its blue/green color, it is used in the manufacture of pyrotechnics.
Health effects / safety hazards: Copper (II) chloride is toxic in high concentrations. It can react with oxidizing agents. The compound is not flammable.
|
Related Links: |
